Aortic stenosis (AS) is a cardiac valvular lesion that can cause sudden death. The prevalence of AS increases with age, including up to 10% of octogenarians [1, 2]. Anesthetic management of patients with AS includes maintenance of preload and systemic vascular resistance (SVR) in an effort to maintain coronary perfusion [3]. Therefore, the use of spinal anesthesia (SA) in patients with AS has been considered a relative contraindication due to the risk of decreasing SVR and a subsequent decrease in preload [4]. Induction of general anesthesia (GA) in patients with AS can also be problematic as induction-triggered hypotension, and positive pressure ventilation can decrease SVR and impede venous return. As such, this patient population presents challenges in selecting the best anesthetic plan.
SA, along with epidural anesthesia, has been shown to reduce morbidity compared to general anesthetics in some studies [5]. A recent systematic review has called into question the notion that SA should be completely avoided in patients with AS; however, prospective trials are lacking [6]. Patients undergoing lower extremity total joint arthroplasty (TJA) frequently receive SA, in accordance with best practice recommendations from the American Academy of Orthopedic Surgeons [7]. Despite the concerns with SA in AS, we have anecdotally observed patients with AS undergoing SA without incident. However, it is unknown if AS patients are at greater risk of adverse outcomes when receiving SA. As a result, the principal aim of this study was to determine the association between outcomes in patients undergoing TJA with AS receiving SA compared to GA. We hypothesized that the incidence of in-hospital mortality and serious complications would be similar between patients with AS who received either GA or SA undergoing TJA.
METHODS
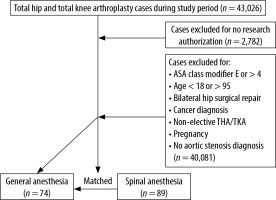
After receiving institutional review board approval, a retrospective chart review was conducted of patients with AS who underwent elective primary TJA between January 1, 2011, and November 30, 2017, at three hospitals within one health system. These patients were identified through International Classification of Disease (ICD)-9/10 codes to identify AS as a medical comorbidity prior to TJA, which was identified by Current Procedural Terminology (CPT) codes for total knee arthroplasty (TKA) and total hip arthroplasty (THA). We then identified all patients with pre-operative AS undergoing TJA. Subsequently, we identified those who received SA using pharmacy data to identify intrathecal local anesthetic administration. We compared outcomes from this group to a matched cohort of patients with AS undergoing TJA receiving GA.
Our inclusion criteria included patients > 18 years old with AS. The diagnosis of AS was based on the most recent American College of Cardiology and American Heart Association guidelines with mild, moderate, and severe AS corresponding to a mean gradient of 20–30 mmHg, 30–40 mmHg, and ≥ 40 mmHg, respectively, with a documented pre-operative echocardiographic report within 12 months of the operation [8]. We excluded patients < 18 and > 95 years old, patients who signed a waiver to exclude their medical records from research studies, American Society of Anesthesiologists physical status (ASA) classification > 4, non-elective THA/TKA, prior aortic valve replacement, and pregnant cases.
Demographic information was collected, including age, sex, height, weight, and body mass index (BMI). Primary endpoints included 90-day mortality, blood transfusion, hospital length of stay (LOS), and the 90-day incidence of deep vein thrombosis (DVT), pulmonary embolism (PE), myocardial infarction (MI), and stroke. Secondary outcomes included the perioperative incidence of unstable arrhythmias.
Statistical analysis
Study demographics and outcomes by receipt of SA or GA were described as frequency and percentages or means and standard deviations. Categorical variables were assessed by Pearson χ2 test and continuous variables were assessed by Kruskal-Wallis test. When counts were below 5, Fisher’s exact test was used. Continuous variables were checked for normal distribution.
We utilized propensity score matching models to determine differences in the occurrence of complications (presence of DVT, PE, MI, or stroke), blood transfusion, mortality within 90 days, and LOS between patients receiving GA vs. SA. Propensity score matching seeks to balance variables of interest between treatment groups in order to reduce bias in estimating treatment effects [9]. In order to detect a moderate effect size (0.35) with 80% power at the 5% significance level, this study needed at least 61 matched pairs. To achieve the best balance in the observed covariates, we used 1 : 1 matching with replacement [10–12]. Patients receiving GA vs. SA were matched on degree of AS severity, patient age, sex, BMI, year of surgery, and procedure type (TKA vs. THA). Parameters were estimated as the average treatment effect on the treated using a logit model and outcomes were exponentiated in order to report adjusted odds ratios and 95% confidence intervals (CIs). Stata/MP 16.1 was used to conduct the analysis.
RESULTS
After applying the inclusion criteria, there were 89 AS patients who received SA and 74 AS patients who received GA for lower extremity TJA (Figure 1). Prior to matching, there were no significant differences in mean age, sex, BMI, left ventricle ejection fraction (LVEF), or type of TJA surgery (TKA vs. THA) (Table 1). In addition, the median LVEF in GA patients was 62% (range 20–75%) compared to 62.5% (range 44–78%) in SA, and the incidence of left ventricular diastolic dysfunction was 41.1% in GA vs. 53% in SA (P = 0.526). However, there were statistically significant differences in the severity of AS between the two groups: in the GA group, 39.2% had mild, 44.6% moderate, and 16.2% severe AS, while in the SA group, 71.9% had mild, 24.7% moderate and 3.4% severe AS (P < 0.001). All SA patients received 0.5% isobaric bupivacaine, with a median dose of 12.5 mg in each independent procedure (TKA, THA). In the SA group, 2 out of 89 (2.2%) patients received invasive blood pressure monitoring, and the arterial line was placed prior to SA in one of them (50%). In the GA group, 18 out of 74 (24.3%) patients received invasive blood pressure monitoring, and the arterial line was placed prior to induction in four (22.2%) of these.
TABLE 1
Demographics: general anesthesia (GA) vs. spinal anesthesia (SA)
Intraoperative fluids, vasoactive medications, and the incidence of hypotension between the two groups are identified in Table 2. The mean volume of intravenous (IV) sodium chloride (NaCl) 0.9% was 1060 ± 418 mL in patients receiving SA versus a mean volume of 706 ± 679 mL in patients receiving GA (P = 0.032). The mean volume of lactated Ringer’s (LR) administered during SA was 1298 ± 852 mL vs. 1839 ± 694 mL in patients receiving GA (P < 0.0001). Additionally, seven patients (8%) in the SA group received intravascular colloid fluids (5% albumin solution) with a mean dose of 459 ± 172 mL versus eight patients (11%) in the GA group with a mean dose of 720 ± 248 mL (P = 0.027). All other variables in table 2 demonstrated no difference between SA and GA groups.
TABLE 2
Intraoperative fluids, vasoactive medications, and incidence of hypotension
Prior to matching, there were no significant differences in complications at 90 days or blood transfusion between the two groups. In the GA group, 3 (4.1%) patients developed complications within 90 days after surgery: 1 DVT, 1 MI, and 1 stroke and MI; in the SA group, 1 (1.1%) patient developed DVT (P = 0.33). Eleven (14.9%) GA patients required blood transfusions during their hospital stay compared with 12 (13.5%) in the SA group (P = 0.801). One (1.1%) patient died in the SA group compared to 0 deaths in the GA group within 90 days of surgery (P = 0.999). There were no inpatient mortalities in either group. The mean hospital LOS in the GA group was 3.2 (1.1) compared to 2.8 (1.4) days in the SA group (P = 0.023).
After propensity matching, all standardized mean differences were less than the guideline maximum differences of 25% (Table 3), and no significant differences between the GA and SA groups remained with regards to the severity of AS, age, sex, year of surgery, BMI, and procedure type (THA vs. TKA) [10]. There were no significant differences in the outcomes of interest after matching (Table 4). Specifically, there were no differences in complications within 90 days of surgery (DVT, PE, MI, and stroke) (GA 2.2% vs. SA 0%; OR: 1.00 [0.95, 1.05], P = 0.233), blood transfusions during hospital stay (GA 12.4% vs. 9% SA; OR: 1.01 [0.86, 1.19], P = 0.751), mortality within 90 days (GA 0% vs. SA 1%; OR: 1.01 [0.98, 1.05], P = 0.498), and hospital LOS (GA mean 3.0 vs. SA mean 2.9, β 0.3 [–0.11, 0.70], P = 0.153). With regards to our secondary outcomes, no patients in either group experienced an intraoperative unstable arrhythmia or were any post-operative unstable arrhythmic clinical events notated.
TABLE 3
Comparison of propensity matched vs. non-matched groups
TABLE 4
Outcomes in general anesthesia (GA)# vs. spinal anesthesia (SA)
DISCUSSION
In this propensity-matched study of patients undergoing TJA with AS, we found a similar safety profile using either SA or GA. No significant differences were found in the incidence of 90-day mortality, serious complications (DVT, PE, MI, stroke), or blood transfusion. The SA group demonstrated a shorter hospital LOS prior to matching; however, no statistical difference persisted after matching.
Best practice guidelines for TJA recommend SA, which some are hesitant to employ in patients with AS [7]. In a systematic review of the use of neuraxial anesthesia (NA) in patients with AS, Johansson et al. [6] found only 10 patients with AS that received NA with 7 non-cardiac and 3 cardiac surgeries. Of the non-cardiac operations, five cases were hip arthroplasty; four received continuous SA, and one received epidural anesthesia (EA). Five out of ten (50%) received invasive arterial monitoring before receiving NA, and all patients remained hemodynamically stable during and after surgery. In contrast, we only found 2.2% of patients in the SA group receiving invasive blood pressure monitoring. Our review finds that more GA patients (24.3%) received arterial line placement compared to SA. Additionally, the average duration of MAP below 20% was slightly longer in GA than SA (75 vs. 70 mins, respectively), although not statistically significant.
Ho et al. [13] retrospectively reviewed the use of hypotensive EA in patients with noncritical, asymptomatic AS undergoing total hip replacement between 1994 to 2005. When hypotensive EA was performed in 22 patients, they reported no complications: no mortality, MI, strokes, nor PE. In our data, we detected no differences in serious complications between SA and GA patients (Table 4). In another study, Taniguchi et al. [14] included 187 patients with severe untreated AS who underwent non-cardiac surgery under GA or SA. At 30 days, eight patients (4.3%) died in the untreated severe AS group. Two of them (25%) received SA, and six received GA. Only 64 of the 187 patients with untreated severe AS underwent hip or knee surgery, and 65 of the 187 received SA. It is not clear from their manuscript which surgeries the 65 SA patients underwent. No intraoperative mortalities occurred. Our results align with these previous studies as we found no intraoperative mortality following TJA in patients with AS undergoing either GA or SA. Our single instance of mortality was in a 40-year-old female with a history of chronic renal failure, kidney transplant, congestive heart failure, severe pulmonary hypertension, and mild-moderate AS who died of unclear reasons on post operative day 4 at home after a TKA.
The safety of continuous SA and combined spinal-epidural anesthesia have also been evaluated in AS. Fuzier et al. [15] reported two cases of severe AS that underwent hip surgery under continuous SA with no subsequent major postoperative complications. Kim et al. [16] reported a case of a 77-year-old female scheduled for lumbar discectomy for L4–L5 spinal stenosis with severe AS. The patient received combined spinal-epidural anesthesia, and no complications were developed during her hospital stay. Lopez et al. [17] reported 2 cases of females with severe AS scheduled for hip fracture repair under continuous SA. Again, there were no hemodynamic complications throughout the cases. We did not have any cases of continuous SA in our data set.
Regarding intra-operative hemodynamics, we found a significantly higher total IV fluids in GA vs. SA (1836 vs. 1464 mL, P = 0.002). GA patients received significantly more ephedrine in GA when compared to SA (30.4 vs. 22 mg, P = 0.050) (Table 2). Phenylephrine doses were higher in SA than in GA (961.5 vs. 586.3 μg, P = 0.401), although the difference was not statistically significant. Nonetheless, there were no significant differences in primary outcomes between both groups after matching. Our data suggest that judicious SA or GA in patients with AS undergoing elective TJA may yield similar results. To our knowledge, the present study is the largest study of propensity-matched patients with AS undergoing TJA under SA versus GA. We were able to quantify the degree of AS and match patients based on whether they had mild, moderate, or severe valvular disease, as well as BMI, year of surgery, sex, and type of joint replacement. There were no in-hospital deaths and, importantly, no intra-operative events. In addition, there was no significant difference in LVEF or left ventricular diastolic dysfunction between the SA and GA groups. Much of the concern regarding the choice of SA or GA in patients with AS is the risk of clinical deterioration on induction of anesthesia. We did not detect any critical intraoperative events surrounding induction in either group.
Our data are subject to the limitations of any chart review, such as missing data or incorrect data entry. Although we used blood pressure as our marker of hemodynamic stability, this is similar to previous studies when evaluating intraoperative outcomes [12]. Additionally, this study is limited by a relatively small sample size (n = 163) and therefore cannot conclude that SA is less or safer than GA. This sample size does allow us to detect a medium effect size of 0.5. However, as the evaluated outcomes are rare events, a larger sample may be needed to identify differences between the groups. Additionally, there are potentially other variables that could be associated with outcomes of interest such as mortality. While we have sought to match on clinically relevant variables associated with outcomes of AS, future studies should seek to further evaluate if other patient or surgical characteristics might additionally contribute to identified outcomes. Despite this, and to our knowledge, this study represents the largest cohort comparing patients with AS receiving SA vs. GA during THA and TKA. Moreover, most of the previous literature has been presented as case reports and lacks a matched comparator. We also did not consider the differential effects of anesthetic technique on delirium, however this has recently been explored in a large randomized clinical trial which did not detect a treatment effect [18]. Lastly, although a larger database would likely identify more patients for a study of AS comparing SA versus GA in TJA, administrative databases would lack information on the severity of AS, intraoperative fluid, and vasoactive medications.
In conclusion, we did not see a significant difference in the incidence of mortality or serious perioperative complications when comparing SA and GA in AS patients undergoing TJA. Although SA has been considered a relative contraindication in patients with AS, this cohort did not demonstrate a difference in complications compared to GA. No intraoperative deaths occurred in either group.