Introduction
Despite the evident tendency of the number of new cases and deaths to decrease, cervical cancer is still one of the most common malignancies in women. Cervical cancer, which, after breast, colon, and lung cancers, is the fourth most common malignancy, is still a global problem [1].
The choice of treatment depends on the stage of clinical advancement assessed according to the International Federation of Gynaecology and Obstetrics (FIGO) staging system. However, the patient’s age, coexisting diseases, results of imaging examinations and the preferences of both the patient and doctor are also considered [2].
The treatment methods of cervical cancer include surgery and radiation therapy as monotherapy or in combination with chemotherapy. In the qualification, which should be based on precise diagnostics, we should avoid the combination of radical surgery and radiation therapy, because these 2 methods significantly increase the frequency and severity of the adverse effects with no influence on the oncological results.
Until the end of 2018, the FIGO staging system used only of a physical examination and an X-ray image (X-ray, radioisotope thermoelectric generator) to confirm hydronephrosis. However, the above assessment was not validated during or after treatment. The 2009 FIGO staging system did not include the of lymph node metastases. This could result in inaccurate evaluation of the progression of the disease, which requires appropriate treatment.
In patients with cervical cancer, the presence of lymph node metastases is a negative prognostic factor. The 5-year patients’ survival with metastatic paraaortic lymph nodes did not exceed 10–25% [3].
In 2018, the FIGO staging system was extended by metastases to the pelvic and paraaortic lymph nodes as well as imaging examinations [4, 5].
Out of all imaging examinations available in the diagnosis of cervical cancer, the positron emission tomography with computed tomography (PET/CT) is considered the most effective method in detecting metastatic lymph nodes [6]. PET/CT is a morphological and important functional imaging method. For this purpose, the technique is based on the information obtained using a positron emitter labelled radiopharmaceutical distributed in the patient’s body. Fluor-18-fluorodeoxyglucose positron emission tomography with computed tomography (18F-FDG-PET-CT) is the marker most commonly used in cervical cancer. For better imaging, a combination of PET and computed tomography CT or magnetic resonance imaging (MRI) is used. The advantages of PET/CT are the ability to perform a full-body examination in a short time with little exposure to radiation and a low risk of adverse effects.
A meta-analysis by Choi et al., estimated the sensitivity and specificity of PET/CT in detecting lymph node metastases in cervical cancer at 82% and 95%, respectively. Both parameters were lower obtained by computed tomography (50% and 92%) and MRI (56% and 91%) [6].
The evaluation of cervical cancer progression is very important to prepare an optimal treatment plan. According Burchardt et al. [7], in up to 30% of patients with cervical cancer the PET/CT may change the original therapeutic plan of radio/radiochemotherapy and surgical treatment. The aim of the study was to compare the diagnostic value of the preoperative evaluation using the 18F-FDG-PET-CT with a histopathological examination of the lymph nodes removed during radical hysterectomy and pelvic lymph node dissection in patients with cervical cancer.
Material and methods
The retrospective study included 49 patients with cervical cancer treated surgically from 1
October 2011 to 31 March 2020. The stage of the disease was determined using the 2009 FIGO classification. The study enrolled patients at stage IA–IB2. The qualification for the treatment included routine laboratory tests, gynaecological examination, ultrasound vaginal examination and 18F-FDG-PET-CT. The basic treatment method applied in all patients was
Piver type III radical hysterectomy and pelvic lymph node dissection up to the level of aortic bifurcation. The analysis excluded 7 patients who had received preoperative neoadjuvant chemotherapy. Forty-two patients were included in further analysis. In all patients, 18F-FDG-PET-CT was performed approximately 2 weeks before the surgery.
The technique of fluor-18-fluorodeoxyglucose positron emission tomography with computed tomography
The 18F-FDG-PET-CT study was performed 60 minutes post-injection (p.i.) of the radiopharmaceutical 18F-FDG with mean activity of 337 ± 69 megabecquerels (MBq), range: 152–544 MBq (administered activity up to 3.7 MBq/kg of body mass). The 18F-FDG-PET-CT study was performed using a Philips Gemini TF16 hybrid scanner (Philips, Cleveland, Ohio, USA). The acquisition protocol included the area of skull apex to mid-thigh (patients laid supine with arms above the head). PET imaging preceded body low-dose CT using the following parameters: 150–245 milliampere seconds (mAs), 120–140 kilovoltage peak (kVp), and pitch of 0.8. The PET section scanning time was 90 seconds. The scanning time did not exceed 35 minutes.
Interpretation of the fluor-18-fluorodeoxyglucose positron emission tomography with computed tomography result
The description of the PET/CT examination considered the medical history, the result of gynaecological examination and transvaginal ultrasound. An experienced specialist in nuclear medicine, who analysed the 18F-FDG-PET-CT scans, was informed about the purpose of the examination. The 18F-FDG-PET-CT result was considered negative when no areas of abnormal 18F-FDG uptake were found. Any other site of the increased 18F-FDG accumulation was described and classified as physiological, malignant, or ambiguous, depending on the size and intensity of tracer uptake. Malignant 18F-FDG uptake was defined as that with an intensity higher than that of the surrounding tissues in the areas non-related to physiology. A PET/CT scan showing at least 1 malignant 18F-FDG uptake site was considered positive. All the remaining areas of increased 18F-FDG uptake were described as benign or unrelated to cancer when at the site of physiological uptake. Any other place that could not be clearly identified was described as ambiguous. PET/CT examinations with all the lesions defined as ambiguous or benign were respectively described as ambiguous or negative.
Pelvic lymph node dissection
All patients were subjected to therapeutic pelvic lymph node dissection up to the level of aortic bifurcation. The procedure was based on a complete removal of the lymphoid tissue from the common iliac vessels, both internal and external, as well as from the obturator fossa and the presacral space. In stage II, a radical Piver type III hysterectomy was performed.
Histopathological evaluation of the lymph nodes
All lymph nodes removed during therapeutic lymph node dissection were examined by an experienced pathologist. The lymph nodes were fixed with 10% formalin and embedded in paraffin. Tissue sections were stained with haematoxylin and eosin. Histopathologically, the nodes were defined as positive (metastatic) or negative (no metastases).
Statistical analysis
The statistical analysis included descriptive statistics presented in the form of mean, standard deviation, median, the range of values for quantitative variables, and frequencies with percentages for categorical variables. Then, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for the PET/CT were calculated in relation to the results of histopathological examination. In the “patient-based” method, the following results were distinguished: true positive – the number of patients with metastases diagnosed usingthe 18F-FDG-PET-CT, and then confirmed by the histopathological examination; false positive – the number of patients with a positive result of 18F-FDG-PET-CT not confirmed by the histopathological examination; true negative – no metastases in the 18F-FDG-PET-CT or in the histopathological examination; and false negative – no metastases in the 18F-FDG-PET-CT, found later in the histopathological examination. In the “region specific” method, the following results were distinguished: true positive – the number of metastatic nodes shown in the 18F-FDG-PET-CT, and then confirmed in the histopathological examination; and false positive – the number of metastatic nodes diagnosed in the 18F-FDG-PET-CT, but not confirmed by the histopathological examination; true negative – the number of all lymph nodes removed in patients with no suspicion of metastasis in the 18F-FDG-PET-CT and then assessed as metastasis-free in the histopathological examination; false negative – the number of metastatic nodes not found in the 18F-FDG-PET-CT, but revealed in the histopathological examination.
Moreover, the McNemar’s test was performed to evaluate the consistency of the results obtained using the 2 methods.
The relationship between the presence of metastases (in the 18F-FDG-PET-CT and histopathological examination) and relapse of the disease was analysed using the Fisher’s exact test.
Results
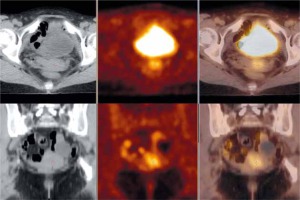
Piver type III radical hysterectomy and therapeutic pelvic lymph node dissection were performed in all patients enrolled in the study, and the 18F-FDG-PET-CT was carried out prior to the surgery. Figure 1 shows an example of the results of the imaging examination.
Fig. 1
Abnormal area of increased fluor-18-fluorodeoxyglucose uptake in the cervix consistent with cancer infiltration. Axial view (upper row) and coronal view (lower row) of the whole body fluor-18-fluorodeoxyglucose positron emission tomography with computed tomography over the pelvis – low-dose computed tomography (CT, left hand side images), positron emission tomography (PET, middle images) and PET/CT fusion (right-hand side images)
The mean age of onset was M = 51.0 years (SD = 11.8). 25.2 (SD = 4.61). The mean time to relapse was M = 19.8 months (SD = 20.3) and the mean follow-up period was M = 37.6 months (SD = 27.7). The mean number of removed lymph nodes removed was M = 28 (SD = 10.2).
According to the 2009 FIGO staging system, 34 patients were classified as IB1 (81.0%). Seven patients (16.7%) were included in the IB2 group, and 1 patient (2.4%) in the IA2 group. None of the subjects were assigned to the remaining groups. As for the histological type, 33 patients (78.6%) had squamous cell carcinoma and 9 (21.4%) adenocarcinomas.
The presence of lymph node metastases in the PET was found in 13 patients (31.0%), while in 12 patients (28.6%) they were reported in the histopathological examination. A relapse was noted in 6 patients (14.3%) (Table 1). Both distant metastases (liver, small intestine, kidney) and local recurrence were found.
Table 1
Patients characteristics
Diagnostic accuracy of the fluor-18-fluorodeoxyglucose positron emission tomography with computed tomography in the detection of metastases to the lymph nodes in patients with cervical cancer
In 8 out of 12 cases, metastases to the lymph nodes were correctly diagnosed using 18F-FDG-PET-CT. The results were confirmed by the histopathological examination. As for the total number of metastatic nodes shown in the PET/CT (N = 20), the findings were confirmed in 9 out of 22 cases (total number of metastatic nodes found in the histopathological examination).
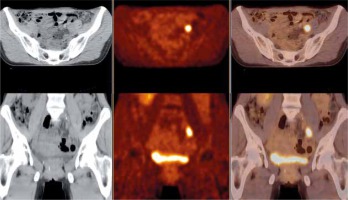
Figure 2 shows an example of the lymph node metastases.
Fig. 2
Abnormal area of increased fluor-18-fluorodeoxyglucose positron emission tomography with computed tomography (18F-FDG-PET-CT) uptake in the left iliac lymph node verified as metastatic. Axial view (upper row) and coronal view (lower row) of the whole body 18F-FDG-PET-CT over the pelvis – low-dose CT (left hand side image), PET (middle image), and PET/CT fusion (right hand side image)
Total sensitivity, specificity, PPV, NPV, and patient-based accuracy were 66.8%, 83.3%, 61.5%, 86.2%, and 78.6%, respectively. In the “region-specific” study, the same parameters reached the following values: 45%, 98.6%, 50%, 98.4% and 58.5%, respectively. Detailed results are presented in Table 2. Figure 3 shows an example of a false negative PET result verified as metastatic lymph nodes.
Table 2
The accuracy of preoperative positron emission tomography with computed tomography in the detection of lymph node meta-stases in patients with cervical cancer
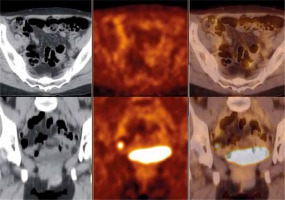
Fig. 3
Bilateral iliac lymph nodes with no fluor-18-fluorodeoxyglucose (18F-FDG) uptake – false negative positron emission tomography (PET) findings verified as metastatic lymph nodes. Axial view (upper row) and coronal view (lower row) of the whole body fluor-18-fluorode- oxyglucose positron emission tomography with computed tomography (18F-FDG-PET-CT) over the pelvis – low dose computed tomography (CT, left hand side image), PET (middle image) and PET/CT fusion (right hand side image)
The McNemar’s test confirmed that the results of the PET and the histopathological examination were consistent in the detection of metastases to the lymph nodes – χ2 (1) = 0.11, p = 0,739.
Metastases to the lymph nodes in the histopathological examination, fluor-18-fluorodeoxyglucose positron emission tomography with computed tomography and the risk of recurrence
No patient diagnosed with metastases to the lymph nodes in the PET/CT, which was then confirmed by the histopathological examination, was diagnosed with recurrence during the follow-up period. On the other hand, in patients with no metastases – true negative (n = 34), the disease relapsed in 6 cases. The analysis conducted using Fisher’s exact test revealed no significance (p = 0.576), and thus no relationship between the variables. Interestingly, after including false positive results of the PET/CT (additionally 5 results, 8 confirmed by the histopathological examination), it transpired that 3 out of 13 patients with a positive result (true or false) had a relapse. In this case, the result of the Fisher’s exact test was still insignificant (p = 0.353).
The relationship between the presence of metastases diagnosed by the histopathological examination (n = 12) and recurrence of the disease also turned out to be statistically insignificant with p = 0.159, both in these patients (n = 0) and those with no metastasis and a relapse (n = 6).
The diagnosis of metastases to the lymph nodes in the histopathological examination and adjuvant treatment
Systemic adjuvant treatment was used in the patients with lymph node metastases found in the histopathological examination.
The study confirmed that most patients diagnosed with lymph node metastases in the postoperative histopathological examination underwent adjuvant treatment. Among 12 patients with lymph node metastases, chemotherapy was applied in 9 cases (75.0%) (Table 3).
Discussion
Cervical cancer most often invades the pelvic lymph nodes. The patients who participated in the study were qualified for surgical treatment, which enabled postoperative histopathological evaluation of these lymph nodes. The complete lymphadenectomy allowed us to quantify the occurrence of metastatic lymph nodes.
The main role of PET/CT in preoperative diagnostics was to exclude metastases to regional lymph nodes to avoid situations in which patients after surgical treatment are qualified for adjuvant radio/radiochemotherapy. Multi-module treatment for cervical cancer not only does not improve treatment outcomes, and it significantly increases the number of complications and treatment costs.
A detailed summary of the study outcomes showed false-negative results of the 18F-FDG-PET-CT in 4 patients and involved the lymph nodes with the size between 0.2 cm and 0.6 cm evaluated in the histopathological examination. In one patient, the preoperative 18F-FDG-PET-CT revealed increased metabolism suggesting a malignant process in one 5 mm pelvic lymph node. In the same patient, the postoperative histopathological examination showed 7 metastatic lymph nodes (the maximum size of 0.7 cm) out of the 37 tested. The results of histopathological examinations confirmed reduced sensitivity of the 18F-FDG-PET-CT in the diagnosis of metastatic lymph nodes depending on the size. According to Kitajimq et al., thesensitivity of the PET/CT in the diagnosis of metastases to the lymph nodes in patients with cervical cancer was 100% for metastatic lymph nodes ≥ 10 mm; 67% for the nodes between 5 and 9 mm and 13% for nodal metastasis ≤ 4 mm [8].
However according to the new 2018 FIGO staging system, the presence of isolated neoplastic cells (diameter < 0.2 mm) or micrometastases (diameter 0.2–2 mm) in the lymph nodes does not change the stage of the disease. This fact should be reported in the medical records [4, 5].
A false-positive PET/CT result was obtained in 5 patients in whom, despite the removal of 16, 34, 43, 41, and 32 lymph nodes, no metastases were found in the postoperative histopathological examination. The largest dimension of a lymph node with the increased glucose metabolism, which arouse a suspicion of metastasis in the PET/CT not confirmed by the histopathological examination, was 17 mm, and the smallest was 5 mm. Three of these patients were diagnosed with a relapse. This confirmed the results published by Chung et al., showing higher rates of recurrence (3/8) in patients with a positive preoperative PET/CT result than in those with negative scans (1/26) [9]. Anyway, this result is interesting, but the reason is not clear-cut and requires further research.
Similarly to the study by Chung et al., we have found higher values of sensitivity and specificity of the PET/CT in the patient-based analysis compared to the region-specific examination. These results are worse than those of the previous studies analysing the PET/CT result alone [10–12]. According to one of the latest meta-analyses of 2018, the total diagnostic sensitivity and specificity of the PET-CT in the diagnosis of lymph node metastases in patients with cervical cancer were as follows: 0.72; 95% CI: 0.69–0.75 and 0.96; 95% CI: 0.96–0.97 [13].
There is also the added value of the 18F-FDG-PET-CT in qualifying for surgical treatment those patients with locally advanced cervical cancer, because the examination provides important information on the possible long-term spread of cancer. In the multicentre study ACRIN 6671/GOG 0233 MS., Gee et al., demonstrated high specificity (98%), PPV (79%) and sensitivity (55%) in detecting distant metastases (non-regional lymph nodes, peritoneal, bone, liver and lung lesions) in patients with locoregional cervical cancer. Distant metastases were unexpectedly diagnosed in as many as 14% of patients [14]. In our study, during preoperative PET/CT examination, distant metastases were not diagnosed in the group of patients with locally advanced cervical cancer qualified for surgical treatment.
According to a meta-analysis conducted in 2019, the diagnostic value of PET/CT in detecting metastases to the para-aortic lymph nodes in patients with cervical cancer showed sensitivity and specificity of 95% CI: 0.54–0.83 and 95% CI: 0.93–0.98, respectively [15].
The recommendations of scientific societies, such as the Polish Society of Gynaecological
Oncology, the European Society of Gynaecological Oncology (ESGO) and the National Comprehensive Cancer Network differ in terms of conditions for extending the clinical examination to include imaging techniques. To sum up, in advanced cervical cancer, pathological verification is needed, both in the presence and absence of suspected metastatic paraaortic lymph nodes. The histopathological verification allows a decision to be made whether radiation therapy should be extended to include this area. This management enables positive lymph nodes to be visualized, as well as avoiding irradiation without clinical indications [16–18].
According to the literature, the presence of metastatic pelvic and paraaortic lymph nodes is one of the most important prognostic factors of high probability of cervical cancer recurrence [19, 20]. In recurrence of cervical cancer, the prognosis is poor because ineffective treatment of the primary lesion indicates a low probability of successful recurrence therapy [21, 22].
The fact that none of the 12 patients with metastatic lymph nodes in the postoperative histopathological examination showed recurrence in the follow-up is the added value of our analysis. Nine patients from this group were treated with adjuvant systemic treatment. Knowing the condition of the lymph nodes in patients with cervical cancer allowed these patients to be subjected to a therapy adequate to the stage of the disease, which translated into treatment results.
At the beginning of our study, the 2009 FIGO staging system, which did not include the evaluation of the lymph nodes, was valid. The new 2018 FIGO classification was extended to include assessment of the lymph nodes, putting emphasis on the importance of metastatic lymph nodes as a major negative prognostic factor in cervical cancer. The diagnosis of lymph node involvement can be made based on a microscopic examination or radiological evaluation [4, 5].
The survival rates of patients with lymph node metastases are significantly worse than in nonmetastatic patients, and therefore detection of nodal lesions is essential for choosing the appropriate treatment and determining the prognosis in patients with cervical cancer. The 3-year survival rate in patients with the absence of nodal metastases during cervical cancer is estimated at 94%, compared to 64% for patients with positive pelvic nodes and 35% for those with positive para-aortic nodes [23, 24].
Until the change of the FIGO staging system in 2018, the results of imaging examinations were not considered when determining the stage of cervical cancer. The sensitivity and specificity of the previously available imaging methods, such as CT and MRI, in the evaluation of lymph node metastases in patients with cervical cancer was low [25–28].
Currently, the preoperative PET/CT performed in patients with cervical cancer allows the stage of the disease to be determined and to choose the appropriate therapy to be chosen.
Nowadays, PET/CT imaging is the most effective method of imaging the lymph nodes [29]. In a retrospective study, Hansen et al., assessed the survival time in 2 groups of patients: the first group had the PET/CT performed before radiation therapy, and the second group was not subjected to such an examination. In the first group, there were significantly improved of 5-year survival and longer disease-free times, which was due to the detection of previously undiagnosed metastatic lymph nodes [6].
The comparison of the results of the imaging examination and the pathological test allowed for an objective evaluation of the effectiveness of PET/CT in the diagnosis of metastatic lymph nodes, and this is of great clinical importance in light of the current 2018 FIGO staging system.
None of the patients with nodal metastases found in the histopathological examination showed a relapse during the follow-up period. Most of this group underwent systemic treatment. However, the findings require further research.
Conclusions
Compared to other imaging methods, the diagnostic effectiveness of preoperative PET/CT examination in detecting metastatic lymph nodes in patients with cervical cancer is high although its accuracy is not explicit.
Despite the usefulness of PET/CT in the diagnosis of lymph node metastases in patients with cervical cancer, in light of the current guidelines and the 2018 FIGO staging system for cervical cancer, false positive results may appear and disqualify patients from surgical treatment.








