Summary
Despite the withdrawal of the ABSORB bioresorbable vascular scaffold (BVS) from clinical use, it is necessary to continue observation of BVS-treated patients. The purpose was to compare the early and long-term outcomes of the BVS with the everolimus-eluting metallic stent (EES) in patients with ST-segment elevation myocardial infarction (STEMI). We found that STEMI patients treated with the BVS showed statistically similar rates of primary and secondary endpoints compared with the EES. However, events of myocardial infarction, revascularization, and stent thrombosis in the BVS group were more frequent.
Introduction
Over the last years, the prognosis of patients in the acute phase of myocardial infarction has improved, but the long-term outcomes are still disadvantageous [1]. The current generation of drug-eluting stent (DES) has demonstrated higher efficacy and safety in the treatment of coronary artery disease in comparison to previous generations of DES and bare-metal stents (BMS) [2]. Despite this, the DES has some limitations. It is well documented that the presence of permanent metallic cages can lead to the enhancement of local chronic inflammatory processes, persistent stimulation of cellular elements, delay of endothelialization, acceleration of neoatherosclerosis, impairment of vessel vasomotion restoration, and mechanical complications [3, 4]. A potential solution of the mentioned issues is the concept of the bioresorbable vascular scaffold (BVS) [5]. Outcomes of the first trials [6–8] of the most widespread BVS (Absorb, Abbott Vascular, Santa Clara, California) in clinical practice were promising. Nevertheless, data from contemporary studies have shown a significantly higher frequency of stent thrombosis (ST) in the BVS-treated patients compared to second-generation everolimus drug-eluting stents (EES) [9]. Based on these reports of an increased risk of scaffold thrombosis, routine implantation of the Absorb BVS was abandoned. Despite the withdrawal of ABSORB BVS from clinical use, it is necessary to continue observation of BVS-treated patients. In the vast majority of studies, patients with ST-segment elevation acute myocardial infarction (STEMI) or with fresh thrombus were excluded from the analyses [6–9].
Theoretically, the advantages of BVS technology may appear appealing to the STEMI population. The physiological advantages of BVS in comparison with the current-generation DES may effectively prevent the occurrence of the no-reflow phenomenon [10]. Moreover, the resorption process with late lumen enlargement, restoration of normal vasomotion, shear stress, and cyclic strain could lead to plaque regression and reduction of late cardiovascular events [5, 11, 12]. Only one randomized trial [13] and a few studies [14–29] were designed to evaluate clinical outcomes in STEMI patients. Nevertheless, the mentioned studies were limited by small sample size or the highly selected nature of the study population.
Aim
The purpose of the present study based on single-center registry was to compare the early and long-term outcomes of real-world patients with STEMI treated with BVS or EES.
Material and methods
Study design
The study included data from the previously published ZABRZE-BVS registry [30]. ZABRZE-BVS, encompassing 456 patients treated with BVS, is an all-comers registry assessing the safety and efficacy of the BVS in the treatment of patients with coronary artery disease in routine clinical practice [30]. Simultaneously in our center the registry including patients treated with EES was conducted. Based on mentioned prospective data registries, we performed a retrospective analysis of consecutive patients with STEMI treated with BVS or EES. The study design was based on intention-to-treat with BVS or EES.
The management in the study population was in accordance with contemporary recommendations of the European Society of Cardiology (ESC) in STEMI [31]. Briefly, during or after transfer to the hospital, the loading dose of acetylsalicylic acid, P2Y12 inhibitor, and weight-adjusted unfractionated heparin were administered. In all cases, coronary angiography with standard techniques and equipment was performed. The decision on the access site (radial, femoral or other) and the type of diagnostic catheters was taken by the operator. Evaluation of the coronary arteries was made based on visual estimation and offline quantitative coronary angiography. If appropriate, the use of intravascular imaging (intravascular ultrasound or optical coherence tomography) was promoted. All therapeutic decisions after coronary angiography, balloon predilatation and postdilatation, use of stents, type of stents, glycoprotein IIb/IIIa receptor inhibitors and other established interventional techniques were at the operator’s discretion.
Along with the new data on the BVS implantation technique, the appropriate management was recommended: 1) accurate measurement of the diameter of the treated segment using quantitative coronary angiography at the maximum extension of the treated vessel (via intracoronary administration of nitroglycerin); 2) optimal preparation of lesion involving the manual thrombectomy and selection of appropriate type and size of the balloon to predilatation with obtaining residual stenosis less than 40% of diameter stenosis; 3) proper BVS implantation technique associated with a gradual increase (2 atm per 5 s) and maintaining the target pressure of the expanded balloon for 30 s; 4) conducting postdilatation with a balloon of the diameter of not more than 0.5 mm from the nominal diameter of the stent. Not mentioned aspects of BVS implantation were consistent with the contemporary state-of-the-art.
After the procedure, the patients were transferred to the intensive care unit. In case of recurrence of ischemia, urgent coronary angiography was performed. After discharge, dual antiplatelet therapy was recommended for at least 12 months. Furthermore, each patient has been prescribed standard secondary prevention in accordance with the ESC guidelines [31].
Data collection
Baseline clinical and angiographic data of enrolled patients were recorded in the institutional database. Information on follow-up, including causes and exact dates of death and cardiovascular events, were obtained from the official registry of the National Health Fund, guaranteeing complete data collection. Therefore, follow-up was available for all patients enrolled in the study.
Endpoints and definitions
Clinical endpoints used in the present study were consistent with the Academic Research Consortium consensus Clinical End Points in Coronary Stent Trials [32]. Briefly, the primary endpoint encompassed the device-oriented cardiovascular endpoint (DoCE) defined as cardiac death, target vessel myocardial infarction and ischemia-driven target lesion revascularization. Secondary endpoints included device success (per lesion), procedural success (per patient), ST and patient-oriented cardiovascular endpoint (PoCE) defined as all-cause death, all myocardial infarction, and all ischemia-driven revascularization. The device success was defined as successful delivery and deployment of the stent at the intended target lesion and successful withdrawal of the delivery system with the attainment of final in-stent residual stenosis of < 20%. The procedure success was defined as achievement of device success in all intended-to-treat lesions without the occurrence of cardiac death, target vessel myocardial infarction, or repeat ischemia-driven target lesion revascularization during the hospital stay. In a multiple target lesion setting, all lesions must meet clinical procedure success criteria to have a patient-level procedure success.
The diagnosis of STEMI was in accordance with the Third Universal Definition of Myocardial Infarction: 1) symptoms of ischemia; 2) the presence of ST-segment elevation consistent with an infarction of ≥ 2 mm in contiguous chest leads, ST-segment elevation of ≥ 1 mm in 2 or more standard leads, or a new left bundle branch block; 3) detection of rising and/or falling of high-sensitive cardiac troponin with at least one value above the 99th percentile of the upper reference limit [31].
Statistical analysis
The comparison of baseline and angiographic characteristics, and early and long-term outcomes between both study groups was performed. Normality of the distribution was verified by the Shapiro-Wilk test. Continuous variables were summarized using the arithmetic mean with standard deviation or median with quartile 1 and 3. Student’s t-test for comparison of continuous parameters with normal distribution was performed, whereas the Mann-Whitney U for parameters with non-normal distribution was used. Categorical variables were compared using Pearson’s χ2 test with the Yates correction if the expected number of observations was less than 5. Additionally, for the primary and secondary clinical endpoints, we used the Cox proportional hazards and logistic regression models to adjust for differences in patients’ baseline characteristics. The factors considered in models were as follows: age, chronic total occlusion in non-culprit lesion, creatinine level on admission, glucose level on admission, hemoglobin level on admission, history of atrial fibrillation, history of diabetes mellitus, left ventricular ejection fraction, male sex, multivessel coronary artery disease, prior myocardial infarction, prior percutaneous coronary intervention, peripheral artery disease, white blood cells on admission. Results were presented as hazard ratio (HR) or odds ratio with a 95% confidence interval (CI). For DoCE and PoCE at 24 months, analysis with the Kaplan-Meier method with the log-rank comparison of curves was performed. A two-sided p-value < 0.05 was considered significant. The Statistica 12 software (StatSoft Inc., Tulsa, Oklahoma) was used for all calculations.
Results
From January 2012 until December 2016, 2,137 patients were hospitalized for STEMI in our center. Of these, 123 patients received the BVS (126 procedures; 163 scaffolds; 151 lesions), whereas in 141 patients the EES (144 procedures; 203 stents; 176 lesions) was implanted. The average age of the study population was 57.7 ±11.8 years, and 78.4% were male. The overall prevalence of diabetes mellitus was 21.6%, mean left ventricular ejection fraction was 43.4 ±8.5%, and the presence of multivessel coronary artery disease was found in 43.2% of patients. Baseline clinical characteristics and risk factors of the study groups are summarized in Table I. Patients treated with BVS were younger, more often had dyslipidemia and less often were obese in comparison to the EES group. Moreover, cardiac arrest on admission was present four times more frequently in the BVS than in the EES group.
Table I
Baseline characteristics of study population
| Factor | BVS (n = 123) | EES (n = 141) | P-value |
|---|---|---|---|
| Age, mean ± SD [years] | 53.8 ±10.1 | 61.0 ±12.2 | < 0.0001 |
| Male, % | 78.9 | 78.0 | 0.87 |
| Arterial hypertension, % | 56.1 | 65.2 | 0.13 |
| Prior MI, % | 12.2 | 17.0 | 0.27 |
| Prior PCI, % | 9.8 | 9.8 | 0.27 |
| Atrial fibrillation, % | 5.7 | 9.2 | 0.28 |
| Diabetes mellitus, % | 21.9 | 21.3 | 0.89 |
| Dyslipidaemia, % | 68.3 | 53.9 | 0.017 |
| Cigarette smoking, % | 37.4 | 43.3 | 0.33 |
| Cardiac arrest*, % | 8.9 | 2.1 | 0.014 |
| Killip class IV*, % | 5.7 | 3.6 | 0.40 |
| eGFR*< 60 ml/min/1.73 m2*, % | 8.5 | 17.7 | 0.030 |
| LVEF*, mean ± SD, % | 43.8 ±8.3 | 43.0 ±8.6 | 0.48 |
| LVEF< 35%*, % | 16.3 | 15.0 | 0.78 |
| Antithrombotic therapy, %: | |||
| Acetylsalicylic acid | 100.0 | 100.0 | 0.99 |
| Clopidogrel | 74.0 | 95.4 | < 0.0001 |
| Ticagrelor | 13.8 | 1.5 | < 0.0001 |
| Prasugrel | 12.2 | 0.7 | 0.0001 |
| Oral anticoagulant | 4.9 | 5.4 | 0.84 |
* On admission. BVS – bioresorbable vascular scaffold, CABG – coronary artery bypass grafting, EES – everolimus-eluting cobalt chromium stent, eGFR – estimated glomerular filtration rate, LVEF – left ventricular ejection fraction, MI – myocardial infarction, PCI – percutaneous coronary intervention.
The analysis of coronary angiography and procedural parameters is presented in Table II. In BVS in comparison with EES patients femoral vascular access and intervention in restenotic lesions were less frequent. The proximal left anterior descending artery was revascularized more often, while the left main and right coronary artery were revascularized less often in the BVS than in the EES group. In the BVS group, the percentage of balloon predilatation was 95.4%, manual thrombectomy was 14.6%, whereas postdilatation was performed in 60.9% of cases. The rates of slow/no-reflow phenomenon were low and similar in both groups.
Table II
Procedural characteristics of study population
Long-term outcomes with adjusted hazard ratios at 12 and 24 months in both analyzed groups are presented in Table III. In Figure 1, the Kaplan-Meier curves with log-rank comparison are shown. The median follow-up period was 931 ±514 days. The primary endpoint at 12 months occurred in 9.7% in the BVS group and 8.5% in the EES group (adjusted HR = 2.61; 95% CI: 0.90–7.56; p = 0.076). At 24 months the incidence of the primary endpoint was 15.2% in the BVS group and 14.9% in the EES group (HR = 2.46; 95% CI: 0.85–7.07; p = 0.095). Occurrence of PoCE was similar at 12 months (13.6% vs. 12.8%; p = 0.16) and at 24 months (21.2% vs. 21.8%; p = 0.25). The percentage of device (95.4% vs. 96.6%; p = 0.82) and procedural success (95.1% vs. 94.3%; p = 0.62) was comparable in both groups. Other clinical outcomes were similar in the BVS and the EES group.
Figure 1
Kaplan-Meier survival curves of 24-month rates of target lesion failure (primary endpoint)
BVS – bioresorbable vascular scaffold, EES – everolimus-eluting stent.
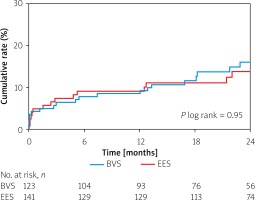
Table III
Long-term outcomes of study population
| Factor | BVS (n = 123) | EES (n = 141) | HR/OR (95% CI) | P-value |
|---|---|---|---|---|
| Primary endpoints: | ||||
| TLF: | ||||
| 12-month, % | 9.7 | 8.5 | 2.61 (0.90–7.56) | 0.076 |
| 24-month, % | 15.2 | 14.9 | 2.46 (0.85–7.07) | 0.095 |
| Secondary endpoints: | ||||
| PoCE: | ||||
| 12-month, % | 13.6 | 12.8 | 1.77 (0.79–3.95) | 0.16 |
| 24-month, % | 21.2 | 21.8 | 1.60 (0.72–3.56) | 0.25 |
| Device success (lesion basis)* | 95.4 | 96.6 | 1.15 (0.34–3.86) | 0.82 |
| Procedural success (patient basis)* | 95.1 | 94.3 | 1.40 (0.36–3.55) | 0.62 |
| Clinical outcomes, %: | ||||
| All-cause death: | ||||
| 12-month | 4.9 | 5.7 | 3.17 (0.72–13.86) | 0.13 |
| 24-month | 7.6 | 10.3 | 2.47 (0.64–9.51) | 0.19 |
| Cardiac death: | ||||
| 12-month | 3.9 | 5.7 | 2.44 (0.52–11.49) | 0.26 |
| 24-month | 6.1 | 10.3 | 1.83 (0.44–7.65) | 0.41 |
| All-MI: | ||||
| 12-month | 7.8 | 5.0 | 1.59 (0.52–4.87) | 0.41 |
| 24-month | 9.1 | 8.0 | 0.90 (0.28–2.88) | 0.85 |
| TV-MI: | ||||
| 12-month | 6.8 | 2.8 | 2.66 (0.69–10.32) | 0.16 |
| 24-month | 9.1 | 4.6 | 1.72 (0.44–6.76) | 0.44 |
| ID-TVR: | ||||
| 12-month | 7.8 | 2.8 | 3.96 (0.99–15.70) | 0.052 |
| 24-month | 12.1 | 4.6 | 3.31 (0.81–13.54) | 0.096 |
| ID-TLR: | ||||
| 12-month | 6.8 | 2.1 | 3.85 (0.83–17.72) | 0.084 |
| 24-month | 10.6 | 4.6 | 2.20 (0.58–8.41) | 0.25 |
| Scaffold/stent thrombosis: | ||||
| Definite* | 4.0 | 1.4 | 5.20 (0.73–36.78) | 0.10 |
| Acute* | 0.8 | 0.0 | –** | 0.94 |
| Subacute* | 2.4 | 0.0 | –** | 0.20 |
| Late* | 0.8 | 0.7 | –** | 0.54 |
| Very late* | 0.0 | 1.1 | –** | 0.95 |
χ2 test with the Yates correction was performed. BVS – bioresorbable vascular scaffold, CI – confidence interval, EES – everolimus-eluting cobalt chromium stent, ID-TLR – ischemia-driven target lesion revascularization, ID-TVR – ischemia-driven target vessel revascularization, HR – hazard ratio, MI – myocardial infarction, OR – odds ratio, PoCE – patient-oriented composite endpoint, TLF – target lesion failure, TV-MI – target vessel myocardial infarction.
During 24-month follow-up, five definite ST in the BVS group (one acute, three subacute, one late) and two definite ST in the EES group (one late, one very late) were observed (HR = 5.20; 95% CI: 0.73–36.78; p = 0.10). Baseline characteristics, pharmacological treatment and procedural results of patients with ST of the BVS group are presented in Table IV.
Table IV
Clinical and procedure characteristics of patients with definite scaffold thrombosis in the BVS group
Discussion
The principal findings of the presented data can be summarized as follows: 1) selection of the BVS during the primary PCI procedure is closely related to the baseline and angiographic characteristics of patients; 2) results show similar rates of DoCE, PoCE, device and procedural success in both groups; 3) there were no statistically significant differences in the incidence of ST and other clinical outcomes. There were no significant differences in the frequency of target vessel myocardial infarction, target vessel and lesion revascularization, and ST between BVS and EES groups. However, they occurred two to three times more frequently in the BVS than in the EES group. Therefore, due to the sample size of our study, the above observations should be interpreted with caution.
A significantly higher frequency of ST in BVS-treated patients compared to second-generation DES in clinical trials was a cause of the withdrawal of ABSORB BVS from clinical use [9]. According to our knowledge, the BVS was implanted in over 150,000 patients on a global scale [30]. Therefore, continuous observation of BVS-treated patients is necessary.
Procedural characteristics
The most common pathophysiological mechanism of STEMI is a ruptured atherosclerotic plaque with a large necrotic core, resulting in delayed healing and chronic inflammation of the vessel [33]. Implementation of BVS technology in this field could lead to potential benefits. The presence of twice as large struts in comparison to the second-generation DES can allow the maintenance of the thrombotic material between the scaffold and the arterial wall [10]. On the other hand, the current state-of-the-art of BVS implantation techniques indicate proper preparation of the lesion by balloon predilatation, which can potentially increase the risk of distal embolization in unstable lesions. The solution of this problem could be the use of manual thrombectomy resulting in the reduction of distal embolization. However, the latest ESC guidelines do not recommend the routine use of thrombectomy in STEMI patients [31]. In BVS registries of ACS patients, the percentage of thrombectomy was from 37% to 84%, whereas direct stenting technique ranged from 53% to 100% [13–29]. In the current study, in almost 25% of patients, evidence of thrombus was found, manual thrombectomy was performed in less than 15%, whereas direct stenting was implemented in less than 5% of cases. Despite a lower frequency of these techniques, the slow/no-flow phenomenon was relatively uncommon and comparable to other real-world registries of STEMI patients [34]. At present, there is a lack of convincing evidence that omission of predilatation is associated with relevant benefits in BVS implantation in the STEMI population.
Adverse events
It has been demonstrated that during BVS implantation there is higher platelet aggregation, stronger adhesion of inflammatory cells, and less intense endothelialisation in comparison to second-generation EES [35]. Furthermore, in STEMI patients, difficult assessment of the reference vessel diameter may result in suboptimal scaffold implantation with incomplete lesion coverage or malposition. These factors could consequently lead to abnormal flow, activation of coagulation factors and thrombocytes, and impaired arterial healing, resulting in a higher risk of both early and late ST [36, 37]. In the present study, the total rate of ST in the BVS group was 4.0%, of which early thrombosis was 3.2%. Late ST was observed in only 1 patient, while there was no occurrence of very late ST. It is noteworthy that the majority of confirmed thromboses occurred in the scaffolds implanted in the first 2 years after introducing BVS technology in our center. In 2 cases of ST, postdilatation was not performed. Moreover, all patients with ST were initially treated with clopidogrel, in most of them resistance to clopidogrel was reported, while 1 patient admitted not to have been using antiplatelet drugs.
In our study, the rate of DoCE was 9.7% at 12 months and 15.2% at 24 months. These outcomes seem to be relatively high in comparison to other studies on the BVS in STEMI [14, 17–23], but similar to large retrospective registries in the STEMI population [1, 2]. It is noteworthy that almost 11% of patients in the BVS arm presented cardiac arrest or cardiogenic shock and 16% had severe systolic dysfunction on admission. These results show a clear reflection of real-world patients. Despite the lack of statistical significance, nearly two to three times higher percentages of target myocardial infarction and target revascularization were observed.
Antithrombotic treatment
Another controversial issue of the BVS technology in STEMI is the choice and duration of appropriate anticoagulant therapy [38]. The duration of dual antiplatelet therapy (DAPT) is a hot topic of discussion in the context of data on late thrombosis in the clinical trials [9]. Analysis of Räber et al. suggested that discontinuation of DAPT after 12 months, scaffold discontinuity and restenosis during the resorption, malapposition and de-endothelialisation may cause the occurrence of very late ST [39]. Regarding the present data, 12 months of DAPT was routinely recommended in all patients in the BVS arm. In general, patients manifested a low risk of bleeding based on a relatively young age, a low percentage of arterial hypertension and chronic kidney disease. Only one-quarter of patients have been prescribed ticagrelor or prasugrel. We did not have post-discharge data on the use of medication and frequency of continuation of DAPT after 12 months. It is also worth emphasizing that in all patients with documented ST, ticagrelor was prescribed and no re-thrombosis was observed. Nevertheless, very long-term observation and pharmacotherapy follow-up data are necessary to fully assess the rate of late scaffold thrombosis.
The percentage of STEMI patients with new-onset atrial fibrillation is estimated as from 6% to almost 21% [40]. Because of the above data, the use of triple antithrombotic therapy in patients after BVS implantation may be associated with an increased risk of bleeding. On the other hand, discontinuation of antiplatelet drugs before 12 months from the discharge may increase the risk of ischemia. Frequently, atrial fibrillation in STEMI is diagnosed after pPCI. There is a lack of data on the use of oral anticoagulation including novel anticoagulants in patients with STEMI after BVS implantation. In our study, triple antithrombotic therapy was prescribed in 7 patients, of which one person used a novel anticoagulant.
Clinical implications
Despite the withdrawal of ABSORB BVS from clinical use, many patients treated with BVS require continuous evaluation in long-term follow-up. The presented outcomes indicate that in the STEMI population the use of bioresorbable scaffolding technology seems to be safe and effective in relation to EES. Nevertheless, further studies, especially with longer follow-up, are necessary. Observations in the STEMI population of patients may translate into better refinement of BVS technology in the future.
Limitations
Due to the retrospective design of the prospective registries data, potential selection biases could occur. We could not verify whether all the patients had been on DAPT for 1 year, as recommended, as there were no data in the registry on DAPT cessation or continuation. The low percentage of intravascular imaging techniques may have been caused by a lack of recommendations during primary PCI or by the gradual introduction of the optimal BVS implantation technique. Low frequencies of the analyzed groups cause that the results of the present study should be interpreted with caution.








