Summary
Expansion of transcatheter aortic valve implantation (TAVI) towards the younger population will result in higher frequency of the procedures in patients having bicuspid aortic valve (BAV). Implantation results in patients with BAV are ambiguous. Different diagnostic methods are used for diagnosis of BAV, various prostheses are being evaluated in this subpopulation and different valve size selection methods are applied. Our study presents the results of TAVI procedures in patients with BAV and TAV, which are diagnosed based on a uniform method – multi-slice computed tomography scans. Only self-expanding valves were used in the study and a uniform valve selection method based on valve annulus measurements was used. Our results show that the use of self-expanding valves in the size selection based on annulus measurements is equally effective in patients with BAV and TAV.
Introduction
A bicuspid aortic valve (BAV) occurs in 0.5–2% of the population [1–3]. This defect predisposes to aortic stenosis, aortic regurgitation, ascending aorta dilatation, and infective endocarditis [3–6]. The incidence of BAV among patients with aortic stenosis depends on patient age. In younger age groups, the incidence is higher in patients qualified for aortic valve surgery and is 59.8% in patients aged 61–70, while it is 25.7% in older patients (e.g., aged 81–90) [7]. According to the transcatheter aortic valve implantation (TAVI) registries, BAV affects 6–8% of patients [8, 9]. Currently, the vast majority of patients referred for TAVI are high-risk surgical patients. Intermediate-risk surgical patients are also referred for TAVI in accordance with the 2017 European Society of Cardiology guidelines [10]. In the coming years, further extension of indications to younger patients should be expected, among whom the BAV incidence will increase. The results of TAVI procedures in BAV patients are ambiguous. Some studies indicate higher early mortality and lower procedural efficacy, while others indicate increased paravalvular leaks and a higher permanent pacemaker implantation rate [1, 3, 11, 12]. Factors that increase difficulties in interpreting results include the method of BAV diagnosis (echocardiography or multi-slice computed tomography (MSCT)), the method of valve selection, as well as the use of different generations of valves and valves with different implantation mechanisms [1, 2, 4].
Aim
The aim of this study is to compare the results of TAVI with the use of a self-expanding prosthesis CoreValve/Evolut R in BAV and tricuspid aortic valve (TAV) patients. The valve size was selected based on measurements of the aortic annulus using MSCT scans.
Material and methods
The TAVI Zabrze Registry is a study collecting data on patients with severe aortic stenosis who were referred for TAVI procedures. The aim of the registry is to monitor treatment outcomes of patients with aortic stenosis in terms of safety, therapeutic efficacy, and cost-effectiveness.
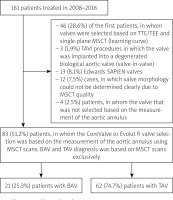
Between November 26, 2008, and December 31, 2016, 161 patients with severe symptomatic aortic stenosis were qualified for TAVI procedures by our Heart Team. The analysis included 83 patients in whom the valve was selected based on the aortic annulus measurements using MSCT scans and in whom CoreValve and Evolut R self-expanding valves were implanted. Valve morphology was determined using MSCT scans. The prostheses were selected by measuring the aortic annulus’s diameter applying the following formula: aortic annulus perimeter in MSCT/π. The valves were selected based on Medtronic’s recommendations. The first 46 (28.6%) patients were found ineligible for analysis because the prostheses were selected based on transthoracic and transesophageal echocardiography and the results of the aortic annulus measurements using MSCT scans in one sagittal plane only. The population of patients was defined as a group of patients on the learning curve. The study excluded 13 (8.1%) patients who received the Edwards SAPIEN valves and 3 (1.9%) patients who underwent a valve-in-valve procedure. The data on 12 (7.5%) patients were excluded from the analysis due to the MSCT scans’ poor quality. Another 4 (2.5%) BAV patients were excluded from the study because the valve was implanted deliberately based on the measurements above the aortic annulus, at the height of valve leaflets. Figure 1 shows how patients were qualified for the analysis.
Figure 1
Flow chard
BAV – bicuspid aortic valve, MSCT – multi-slice computed tomography, TAV – tricuspid aortic valve, TAVI – transcatheter aortic valve implantation, TEE – transesophagea1 echocardiography, TTE – transthoracic echocardiography.
Before the procedure, the patients gave their informed consent to the proposed treatment. The patients were divided into groups based on valve morphology: group I consisted of 21 (24.7%) BAV patients, and group II consisted of 62 (74.7%) TAV patients.
MSCT examination and measurements
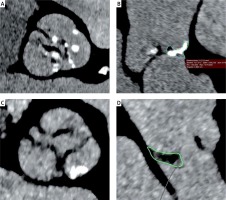
The MSCT examination was performed according to the previously described protocol [13]. Measurements of the aortic annulus, roots, and aorta were performed using OsiriX Pro software (by Pixmeo SARL, Switzerland). Aortic annulus measurements comprised: minimum size, maximum size, perimeter, and area of the annulus. The aortic annulus diameter obtained from aortic annulus perimeter measurements was used to select the prosthesis. Oversizing was defined as the perimeter’s ratio at the base of the implanted valve to the perimeter of the native valve annulus according to the following formula: valve perimeter/annulus perimeter × 100% – 100. The diagnosis of BAV was made based on the MSCT examination, according to the Sievers and Schmidtke classification [14]. Type 0 was diagnosed when two fully developed cusps were present with no raphe and one commissure (true BAV); type 1 when one fully developed cusp was present with two smaller cusps joined with one raphe; and type 2 when two raphae were present (Figures 2 A, B). Functional BAV was diagnosed when three more or less symmetrical cusps were present with no raphe, but their secondary fusion was present due to degeneration of the valve (Figures 2 C, D). Each raphe or cusp fusion was analyzed in three perpendicular planes. The analysis consisted of shifting the intersection plane parallel to the annulus from the left ventricular outflow tract towards the aorta to the top of leaflets and shifting the plane perpendicular to the raphe/the fusion from the center of the aortic valve to the circumference. In 3 cases, the BAV analysis was supported by MSCT image sequences at different R-R intervals: 10%, 20%, 30%….100%. For this purpose, a Somatom Definition Flash (Siemens Forcheim Germany) was used.
Figure 2
A – Bicuspid aortic valve with raphe type 1 L/R. B – Raphe with calcification in patient with bicuspid aortic valve type 1 L/R. C, D – Functional bicuspid aortic valve with fusion of the valve leaflets L/N
TAVI procedures were performed in a catheterization laboratory or a hybrid operating room. Seventy-one (85.5%) CoreValves and eleven (13.3%) Evolut R valves (Medtronic, Minneapolis, MN, USA), in sizes 26, 29, 31, were implanted. All procedures were performed routinely, as described elsewhere [13, 15, 16].
Angiographic assessment
After valve implantation, the paravalvular leak was assessed angiographically based on the Sellers classification of aortic regurgitation [17]. A contrast agent (15–20 ml) was administered at 10 ml/s 450 PSI. The evaluation was carried out independently by three experienced interventional cardiologists. A common position was agreed upon in case of discrepancies. Seventy-seven aortographies were analyzed after valve implantation. In 6 (7.23%) patients, aortography was not performed, mainly due to renal failure.
Composite endpoints were adopted according to the criteria of the Valve Academic Research Consortium (VARC 2), i.e., device success, early safety (at 30 days), and clinical efficacy (1-year evaluation) [18].
The patients were evaluated during the hospital stay, 30 days, and 6–12 months after the procedure at the cardiology outpatient clinic.
Statistical analysis
The results obtained are presented as a standard deviation and mean value. The Kolmogorov-Smirnov test was used to evaluate the compatibility between distributions and a normal distribution. Student’s t-test was used for dependent and independent variables when the pair’s distribution was similar to the normal distribution. Yates’s χ2 test and Fisher’s exact test were used for assessing differences among categorical parameters. The Kaplan-Meier analysis enabled us to determine survival. The log-rank test was used to compare survival curves. The value of p < 0.05 was considered statistically significant. All calculations were done using Statistica 10 software (TIBCO Software Inc., Palo Alto, California).
Results
Baseline characteristics of patients are shown in Table I. Patients did not differ significantly in terms of basic clinical, echocardiographic, and MSCT parameters.
Table I
Baseline characteristics
[i] Data are shown as numbers (percentage) or mean ± standard deviation. AVA – aortic valve area, AVPG – aortic valve pressure gradient, BAV – bicuspid aortic valve, BMI – body mass index, BSA – body surface area, CABG – coronary artery bypass grafting, COPD – chronic obstructive pulmonary disease, CRT-D – cardiac resynchronization therapy with defibrillator, DDDR – dual chamber rate adaptive pacemaker, ICD – implantable cardioverter-defibrillator, LVEF – left ventricular ejection fraction, MI – myocardial infarction, MSCT – multi-slice computed tomography, NT-pro BNP – N-terminal pro-B-type natriuretic peptide, NYHA – New York Heart Association, PCI – percutaneous coronary intervention, TIA – transient ischemic attack, TAV – tricuspid aortic valve, VVIR – single chamber rate adaptive pacemaker.
According to the Sievers and Schmidtke classification, none of the patients had type 0, 13 (61.9%) had type 1, 3 (14.3%) had type 2, and 5 (23.8%) had functional BAV.
The results of TAVI treatment are presented in Table II. Second valve implantation was necessary in 2 patients of the BAV group (due to valve embolization to the ascending aorta) and 2 patients of the TAV group (in 1 patient due to valve embolization and the second one due to very low implantation associated with a severe paravalvular leak).
Table II
Procedural and postprocedural data
[i] Data are shown as numbers (percentage). AVG – aortic valve gradient, BAV – bicuspid aortic valve, CRT-D – cardiac resynchronization therapy with defibrillator, DDDR – dual chamber rate adaptive pacemaker, EOA – effective orifice area, ICD – implantable cardioverter, LVEF – left ventricular ejection fraction, PVL – paravalvular leak, TAV – tricuspid aortic valve, TAVI – transcatheter aortic valve implantation, TF – transfemoral approach, TS – trans-subclavian approach, TAo – direct aorta approach, VVIR – single chamber rate adaptive pacemaker.
Valves were not implanted in 2 patients. In 1 BAV patient, the venous graft was damaged during transaortic access as part of the sternotomy, and symptoms of myocardial infarction occurred. Coronary artery bypass grafting was performed, and TAVI was postponed. The patient died on postoperative day two. During subclavian access in 1 TAV patient, after valvuloplasty, symptoms of ischemia appeared, leading to cardiogenic shock with cardiac arrest. The patient died despite resuscitation before valve implantation.
After TAVI, comparable results were obtained in both groups. The groups did not differ in terms of effective orifice area (EOA), mean aortic valve gradient, or left ventricle ejection fraction. In the echocardiographic assessment, 3 (14.28%) BAV patients had a moderate paravalvular leak (patients with CoreValve). Six (9.67%) TAV patients had a moderate paravalvular leak (patients with CoreValve). In the angiographic assessment of paravalvular leak, according to Sellers, no significant differences between those two groups were found. Grade 3 paravalvular leak in the angiographic assessment occurred only in 1 (4.7%) BAV patient (CoreValve prosthesis). There was no grade 3 paravalvular leak in any TAV patient (Table II).
During the hospital stay, 4 (19.05%) BAV patients required permanent pacemaker implantation. In the TAV group, 15 (24.2%) patients (p = NS) needed permanent pacemaker implantation (Table II).
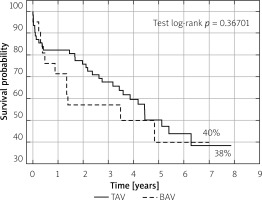
Patients from the two groups did not differ significantly in terms of device success, early safety, clinical efficacy (1-year evaluation according to VARC 2 criteria) or individual components of these composite endpoints. The results are shown in Table III. Kaplan-Meier survival curves demonstrated similar survival probability at long-term observation (Figure 3).
Table III
Composite endpoints
Discussion
The results of TAVI treatment of BAV patients are presented in comparison with TAV patients. It is a single-center study, using one type of transcatheter prosthesis and a homogeneous procedure for valve selection (annular sizing). A uniform way of BAV diagnosis – based on MSCT scans – was also applied.
The classification of BAV used in the study refers to the full spectrum of morphologies presented in the material of BAV by taking into account the Sievers classification and the presence of functional BAV. The Sievers classification is a surgical/anatomopathological classification, and the recently introduced Jilaihawi classification is based on MSCT studies. We did not use Jilaihawi’s classification because it does not take into account the valves with two raphae, which are defined by Sievers as type 2. In our material, there were three such patients. In turn, in Jilaihawi’s classification, there is a three-commissural type, which, according to this classification, corresponds to the concept of an acquired, functional BAV [19]. This type of BAV does not fit into the Sievers classification. Our material included 5 patients with functional BAV, which concerns valves with three symmetrical leaflets, but two leaflets are fused at the commissures (Figures 2 C, D). In the literature, there is a concept of a functional BAV in which the adjacent leaflets are fused but are not underdeveloped [19, 20]. The morphologies of BAV differ very much on CT examination, and not all correspond to the classical Sievers classification. The classification we use is not perfect; however, it reflects the different morphologies of BAV. Such a division exists in the literature, e.g., in the publication by Kochman et al. [21].
TAVI procedures in BAV patients may be associated with numerous technical difficulties. The factors which increase the difficulty of TAVI procedures in BAV patients include the number of eccentric calcifications, larger annulus diameter, aortic bulb and ascending aorta, longer cusps, lack of consensus concerning the use of annular and aortic valve measurements in valve selection [2–6, 10, 22]. It is uncertain whether the same valve selection methods should be used for bicuspid and tricuspid morphology.
In our study population, BAV occurred in 24.7% of patients. Its prevalence is, therefore, much higher than that reported by the European registries, where it ranges from 2.7% to 6.7%, but lower than in the Chinese population, where its prevalence in patients who have undergone TAVI is 37.1% [2, 11, 23, 24]. The prevalence of BAV in our study is similar to the observations of Roberts et al. [7]. Their observations are based on surgical aortic valve replacement in patients with aortic stenosis and on the analysis of excised aortic valves. The analysis does not include patients with rheumatic aortic valve stenosis. In the study of Sievers and Schmidtke [14], based on reports from 1,206 surgical aortic valve replacement, BAV was diagnosed in 33.9% (409) of patients with aortic defects: stenosis, regurgitation, and combined aortic defect. Perlman et al. diagnosed BAV based on MSCT examination and found it in 12% of patients who underwent TAVI [20]. In most cases, Jilaihawi et al. diagnosed BAV on MSCT scans and confirmed a significant discrepancy in the prevalence of BAV [19]. On average, BAV was found in 2.5% of patients; however, in the Italian center participating in the study, it was 0.32%, and in the Chinese center it was up to 66.7% [19].
The percentage of patients with BAV will increase with the decrease in the age of patients eligible for TAVI. Roberts et al. found that in the group of patients aged 61–70, the rate of those with BAV is 59.8% [7]. The high percentage of BAV patients in our study may indicate underestimating the percentage of BAV patients in the registries and observational studies. On the other hand, it means a frequent treatment of BAV patients without awareness of the presence of this malformation. The high percentage of BAV patients in our study is also due to its diagnosis with MSCT scans. The superiority of MSCT examination to echocardiography was proven by Tanaka et al., who stated that the use of MSCT examination has 94.1% sensitivity and 100% specificity regarding the diagnosis of BAV [25]. The analysis based on echocardiography showed that 20% of BAV patients are not identified. In our study, we were not able to define the valve type based on MSCT scans in 12 (7.4%) patients (Figure 1). Kim et al. found that in 16/217 (7.4%) BAV patients, the BAV phenotype could not be defined [26]. The groups of patients included in our study were well balanced. In our study, comparable results were obtained among BAV and TAV patients, assessed on the basis of VARC criteria. Good results were also obtained in terms of annual survival (Figure 3).
Our 30-day and 1-year mortality results are comparable to other studies (Table IV) [24–33]. The differences regarding mortality and other complications such as life-threatening bleeding could be explained by the qualification of patients in our study with a higher risk of surgical treatment and numerous comorbidities, and the use of first-generation valves in most cases. The high 1-year mortality rate also reflects the complex profile of patients qualified for TAVI in Polish centers in the examined years, related to financial constraints and access to treatment.
Table IV
30-day and 1-year mortality across the studies
| Study | 30-day mortality | 1-year mortality | ||
|---|---|---|---|---|
| BAV | TAV | BAV | TAV | |
| Himbert et al. 2012 [24] | 6.6% | |||
| Hayashida et al. 2013 [2] | 4.8 % | 8.2 % | ||
| Mylotte et al. 2014 [3] | 5.0% | 17.5% | ||
| Kochman et al. 2014 [27] | 4.0% | 7.0% | 18% | 17% |
| Bauer et al. 2014 [23] | 11.0% | 11.0% | 13.0% | 20.0% |
| Costopoulos et al. 2014 [11] | 14.0% | 4.0% | 32.0% | 14.0% |
| Yousef et al. 2015 [28] | 8.3% | 16.9% | ||
| Perlman et al. 2016 [20] | 3.9% | |||
| Jilaihawi et al. 2016 [19] | 3.8 % | |||
| Yoon et al. 2017 [22] | 3.7 % | 3.3% | 11.4% Early-generation devices: 14.5% New-generation devices: 4.5% | 11.2% 13.7% 7.4% |
| Sannino et al. 2017 [29] | 3.4% | 3.1% | 8.5% | 10.5% |
| de Biase et al. 2018 [30] | 5.0% | 3.0% | ||
| Tchetche/de Biase et al. 2019 [31] | 0.0% | 3.4% | ||
| Makkar et al. 2019 [32] | 2.6 % | 2.5% | 10.5% | 12.0% |
| Forrest et al. 2020 [33] | 2.6 % | 1.7% | 10.4% | 12.1% |
There were no differences in the frequency of permanent pacemaker implantation or the prevalence of moderate/severe paravalvular leak. Furthermore, the two study groups did not differ in terms of the mean transvalvular gradient in echocardiographic and hemodynamic measurements immediately after the procedure. A relatively high percentage of permanent pacemaker implantation was found in our patients. This is probably associated with the rare use of fully repositionable Evolut R valves in the study.
BAV patients have not been eligible for randomized trials so far [34–37]. Other non-randomized, observational studies concerning the comparison of TAVI procedures in BAV and TAV patients yielded ambiguous results. Bauer et al. found a higher prevalence of aortic regurgitation in BAV patients [23]. Costopoulos et al. discovered that TAVI in BAV patients is characterized by higher early mortality and lower procedure efficacy [11]. On the other hand, de Biase et al. demonstrated that in the group of BAV patients, TAVI-in-TAVI procedures should be performed more frequently, indicating lower efficacy of TAVI procedures in BAV patients [30]. Hayashida et al. stated that TAVI procedures in BAV patients gave the same results as in TAV patients [2]. Meanwhile, Yoon et al. reported comparable results, but with newer valve generation only [22]. The use of older generation valves was associated with lower efficacy of the procedure, mainly due to more frequent conversion to surgery, the need to implant a second valve, and more frequent PVL; different valves, ways of diagnosing BAV, and methods of valve selection were applied. Two recent observational studies on TAVI procedures in BAV and TAV patients employed propensity score matching. Makkar et al. did not find differences between BAV and TAV patients in terms of 30-day and 1-year mortality. They found a significantly higher risk of stroke during the 30-day observation, a higher risk of annulus rupture, conversion to surgery, permanent pacemaker implantation, and a higher incidence of a severe paravalvular leak in BAV patients after TAVI. Third-generation balloon-expandable valves were used in this study [32]. Forrester et al. did not find differences between BAV and TAV patients in terms of total mortality, stroke, or valve hemodynamics. Evolut R and Evolut Pro self-expanding valves were used in this study [33]. The authors demonstrated a longer procedure time and a higher rate of reoperations on the aortic valve in BAV patients [33]. Following this study, Medtronic received a CE mark to indicate the Evolut R valve in intermediate, high, and extremely high-risk BAV patients. The population in our study is slightly different from those in the registry in terms of BAV prevalence, less frequent history of myocardial infarction, coronary intervention, and CABG in BAV patients.
In our view, valve selection plays a decisive role in the treatment outcomes of BAV patients. The rules relating to the methods of valve selection have not been established yet; however, several methods have been proposed. Annular sizing is the most common one, but other valve selection methods are also possible, such as balloon sizing, 1- or even 2-size downsizing, sizing based on inter-commissural distance, as well as increasingly popular supra-annular sizing [26, 31, 38]. Doubts arise as to the fact that the bicuspid aortic valve perimeter at the margin height is approximately 2/3 of the annulus circumference and that three types of aortic valve implantation landing zones (tube, flare, and taper) emerge from measurements of the annulus diameter and inter-commissural distance [25, 31]. In our study, the valve selection was based on the annulus perimeter measurements taken during MSCT examination. Firstly, this conviction stems from the fact that the authors obtained good results in reducing PVL using valve selection based on annulus perimeter measurements obtained from MSCT scans (in comparison to echocardiography) [39]. Secondly, it also results from the fact that the CoreValves and Evolut R valves were designed to be placed on the annulus, not to be anchored at the height of the cusps or the inter-commissural distance. In addition, the CoreValves and Evolut R valves are of a tapered shape in cross-section at the base. Valve implantation based on the annular sizing method results in selecting larger valves, which yields a larger effective orifice area and a lower gradient. A higher gradient after valve implantation, often due to valve deformation with BAV, can be reduced by post-dilatation, thus changing the valve’s shape to a more circular one at the level of the native margins of the valves, which improves the mobility of the cusps and prolongs the valve’s durability. The supra-annular sizing method was applied by the authors in 4 patients excluded from the high-risk cardiac surgery treatment due to very large aortic valve annuli (> 30 mm) and the proctor’s suggestion. Those patients’ data were deleted from the analysis so that the study group could be treated in the same way.
Only one type of valve was used in the study, initially because of the conviction that the balloon-expandable valves should not be used in bicuspid valves due to the more frequent elliptical shape of the annulus, which could cause the valve deformation and worsen its performance. It could impact the durability of the prosthetic valves. Philip et al. reported that the annulus in BAV valves had mostly a round shape [40]. This is confirmed by the measurements taken in our study. The annuli did not differ significantly in terms of minimal and maximal diameter and the degree of ellipticity. There are some reports regarding the beneficial effects of BAV patients’ treatment using balloon-expandable valves [22, 32]. There is a different selection method for each valve type – based on the annulus diameter and radial force of a given valve – each of them (self-expanding, balloon-expandable, and mechanically expandable valves) has a different optimal degree of oversizing, specified by the manufacturer). Comparing TAVI procedures in BAV and TAV patients, if different valve types are applied, it may lead to incorrect conclusions. In the future, it is advisable to compare the treatment results of BAV patients among patients in whom different valve selection methods have been applied in order to assess which one is optimal for BAV patients.
Limitations of the study: The study has many limitations due to its retrospective nature and the fact that it is a single-center study with a small number of patients. The small number of patients could have contributed to the lack of clinical and anatomical differences between the studied groups. Another limitation is the lack of analysis of MSCT scans by a dedicated, independent core-lab in terms of valve morphology, mainly because in our study, the BAV percentage was higher than in other studies. In the case of 25% of our patients, BAV was described as functional, which is usually associated with less calcification at the site of leaflet commissures. This could have impacted the results. Although three experienced interventional cardiologists independently assessed paravalvular leaks after TAVI, the fact that this parameter was not evaluated in dedicated core-labs should be considered another limitation of this study. It is now believed heavily calcified BAV and/or with long heavily calcified raphae are prone to worse PVL grade and clinical outcome. However, the aim of the study was not to analyze the calcification volume and its impact on PVL. Furthermore, the use of first-generation valves in most patients could be viewed as a limitation as well.
Conclusions
In the short- and medium-term, the treatment of patients with severe aortic stenosis and the bicuspid aortic valve is as effective as that with the tricuspid aortic valve using self-expanding TAVI valves when the valve size selection is based on annular sizing using MSCT scans. However, these results require confirmation in studies on a larger population and studies comparing different prosthesis selection methods in BAV patients.