Introduction
Breast cancer is the most prevalent type of cancer and the most lethal and recurrent cause of death. Invasive ductal papillary cancer is the subtype and most common type of breast cancer, and it comprises approximately 80% of all cases [1, 2]. Despite advancements in diagnostic and treatment options, breast cancer-related death is still high. The main reason for this intriguing development was due to the intra-tumour biological heterogeneity and the subtype of tumour diversity [3]. Invasive ductal cancer neoplasia originates in the milk ducts of the breast tissue and then begins to spread invasively throughout the surrounding fibrous stroma [4].
The main reason papillary lesions of the breast are heterogeneous is due to benign, atypical lesions and noninvasive and invasive malignant subtypes. Still, there might be confusing overlapping features, even though it is straightforward to diagnose typical histological features in papillary cancer [5]. In light of the available data, the comparison of invasive ductal carcinoma and papillary carcinoma reveals more favourable outcomes in prognosis and survival of papillary carcinoma [6]. In mammography, papillary cancer is the most prevalently detected and recognized cancer. The condition is usually seen as a soft tissue mass with calcification, which accounts for less than half of the cases. The calcification pattern is changeable. Sometimes, invasive ductal carcinoma can be confused with a pleomorphic calcification pattern. Papillary cancer is usually seen as an oval-shaped, solid mass with a cystic component that dilates the duct by showing intraductal localization on ultrasonography [7].
Material and methods
This retrospective observational study was performed in the radiology department at Dicle University Medical School’s Training and Research Hospital between January 2010 and March 2019. The institutional review board approved our retrospective study. Overall, 88 patients were included in the study. The patient group of this study consisted of 44 patients suspected of having malignancy in the breast by mammography or ultrasonography, detected to have solid breast mass, or who underwent Tru-Cut tissue biopsy, were obtained from the hospital patient database management system. The control group of this study consisted of 44 patients who were diagnosed to have invasive ductal cancer.
Ultrasonography (US) was performed by 2 different radiologists in unblinded settings using a Toshiba™ Applio™ 500 device, with a 6–12 MHz linear transducer. After bilateral breast and axilla examination, ultrasonographic images previously transferred to the Picture Archiving and Communication System (PACS) were evaluated. MG examinations were obtained using the IMS Giotto Class (IMS, Bologna, Italy). Images were taken in craniocaudal and mediolateral oblique planes on mammography, and tomosynthesis images were evaluated via the PACS.
Histopathologic evaluation was done on the patients who had a previous true tissue biopsy from lesions with the suspicion of malignancy. After determining histologically diagnosed lesions retrospectively, the lesions were evaluated by ultrasound and mammography according to contour and shape features, internal structure, relationship with the surrounding soft tissue, the orientation of the long axis concerning the skin, posterior acoustic changes, and calcification-cystic component. In addition, radiologic features of specimens that were diagnosed as invasive ductal carcinoma or papillary carcinoma were evaluated in light of these characteristics. Histopathological results were accepted as the gold standard. The lesions’ ultrasonographic and mammographic features were evaluated and compared using the PACS. Radiologic features were compared and evaluated using SPSS v21 program.
Statically analysis
All analyses were performed in SPSS v21 software. Compliance with the normal distribution for the age variable was checked using the Shapiro-Wilk test. All analyses were performed in SPSS v21 program. Compliance with the normal distribution for the age variable was checked using the Shapiro-Wilk test. Comparison of the age variable that did not fit the normal distribution between the groups was done using the Mann-Whitney U test. Intergroup evaluation of categorical variables was done using Pearson χ2, Yates corrected χ2, and Fisher’s exact test, whichever was appropriate. Prospective selective logistic regression analysis was performed using variables with a statistically significant difference to determine the most effective variables in determining papillary carcinoma. The performance measurement of the model obtained after the logistic regression analysis was calculated. Evaluation of model performance was made using the receiver operating characteristic (ROC) curve. P-values < 0.05 were considered statistically significant.
Results
The study was conducted on 88 patients. Eighty-seven of them were women, and there was only one man. The patient’s mean age was 48.40 ±13.41 years. The patient group comprised 44 patients with papillary breast carcinoma, and the study group was composed of 44 patients with invasive ductal cancers. There was no statistical difference between the groups regarding age (p = 0.825). Descriptive statistics for the age variable are shown in Table 1.
Table 1
Descriptive statistics of age variable
| Parameters | Average | SD | Mean | Smallest | Largest | p |
|---|---|---|---|---|---|---|
| Invasive ductal Carcinoma | 47.86 | 10.75 | 46.00 | 31.00 | 78.00 | 0.825 |
| Papillary carcinoma | 48.93 | 15.75 | 44.50 | 22.00 | 80.00 | |
| Total | 48.40 | 13.41 | 45.50 | 22.00 | 80.00 |
While all patients with invasive ductal carcinoma were female, one patient (2.27%) in the papillary carcinoma group was male. There was no statistical difference between the groups regarding gender distribution (p = 1.000). The most common tumour localization was the upper outer quadrant with 68.92%, followed by the retrocaval area with 17.57%. There was no statistical difference between the groups in terms of tumour location (p = 0.13).
While irregular contour was observed in the mammography of all patients in the invasive ductal carcinoma group, smooth contours were observed in 4 (22.22%) patients in the papillary carcinoma group. The difference was found to be statistically significant (p = 0.007). There was no statistical difference between the groups regarding the presence of microcalcification in mammography (p = 0.904).
While smooth contour was observed in 2 patients (4.55%) on U/S in the invasive ductal carcinoma group, smooth contour was observed in 18 patients (40.91%) in the papillary carcinoma group. This difference was also found to be statistically significant (p < 0.001). In addition, the frequency of lobulated appearance on U/S in papillary carcinoma was statistically higher than in the other group (p = 0.004).
In both groups, at least half of the patients did not show any changes in posterior acoustics on U/S. Shadowing was observed in 43.8% of patients with invasive ductal carcinoma. While the posterior acoustic was bright in 25.00% of papillary carcinoma patients, a mixed appearance was observed in 2 (4.55%) patients (p = 0.002). When the internal structure was evaluated on U/S, a homogeneous appearance was observed in most patients with papillary carcinoma (75.00%). In comparison, this rate was found to be 22.73% in patients with invasive ductal carcinoma (p < 0.001). There was no statistically significant difference between the groups in terms of echogenicity, orientation, presence of echogenic halo, and presence of cystic component (Table 2). The distribution of the contour evaluation results in U/S according to the groups is shown in Figure 1, and the distribution of the posterior acoustic evaluation results in the U/S according to the groups in Figure 2.
Fig. 1
Greyscale sonography and RDUS image showing a homogeneous, solid mass lesion with smooth lobule contour and arterial, and venous blood supply, causing acoustic augmentation in the posterior. In the craniocaudal mammographic examination, radiopaque lesions in the outer quadrant in a certain ductal trace with lobulated contours with sharp borders and continuing with each other were shown
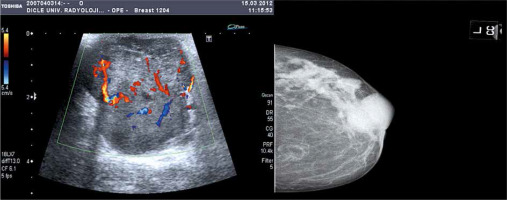
Fig. 2
Greyscale sonography and RDUS image showing a prominent hypoechoic solid mass lesion with irregular spicule contours, with the echogenic halo of desmoplastic reaction. In the mammography examination in the craniocaudal plane, a nodular, radio-opaque lesion with spicule contours was observed in the retroareolar area
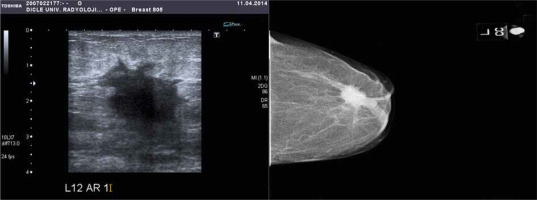
Table 2
Distribution of patient and tumour characteristics by groups
A statistically significant difference was detected between the groups in terms of mammography contour, U/S contour, U/S shape, U/S posterior acoustics, and U/S microstructure. A prospective selective logistic regression analysis was performed to determine which of these variables was the influential factor.
Logistic regression analysis showed that the presence of homogeneous appearance in the internal structure on U/S (p < 0.001) and the absence of shadowing in the posterior acoustics on U/S (p = 0.001) were the most pertinent findings in detecting papillary carcinoma.
A tumour with a homogeneous internal structure is 16,869 times more likely to be papillary carcinoma than invasive ductal carcinoma, according to the U/S analysis. Also, the same probability was found to be 0.101 times less for a tumour with posterior acoustic shadowing on the U/S analysis (Table 3). When we evaluate the performance of the prediction model in our study, the sensitivity of this model in detecting the presence of papillary carcinoma was 61.40%, the selectivity was 90.90%, the positive predictive value was 87.10%, the negative predictive value was 70.18%, and the correct classification rate was 76.14%. The success of the model in detecting papillary carcinoma was found to be statistically significant (p < 0.001) (Table 4).
Table 3
Logistic regression analysis results
Table 4
Patient classification success and performance criteria of the logistic regression model
Discussion
Papillary carcinomas are low-grade breast cancers, and their prognosis is much better than invasive ductal carcinoma. They constitute approximately 0.3–2% of all breast tumours. Papillary carcinomas are usually detected in people’s mid-40s and 60s. The condition is most prevalent in women [8, 9], although it can rarely be detected in males. Indeed, our findings in this study were consistent with the reported data in the literature, and the mean age for papillary carcinoma was 46 years. In addition, the number of female patients was much greater in our study, and only one male patient had papillary breast carcinoma. On the one hand, invasive ductal carcinoma defines all invasive cancers that do not show any specific type of morphology. On the other hand, the diagnosis of invasive ductal carcinoma is usually made after eliminating all other types of cancer. For this reason, invasive ductal carcinoma diagnosis is usually made after eliminating all other possible types of cancer. This type of breast cancer usually originates from the ductal epithelium and comprises approximately 50–75% of all cancers. Similarly to papillary breast carcinoma, invasive ductal carcinomas are seen much more frequently in women, while the most common prevalent age is between the late 40s and 60s [10, 11]. Similarly, our findings were similar to other reports in the literature.
Morphologically, ductal carcinomas show an intense stromal reaction but have a hard consistency and infiltrate into the environment. Histologically composed of different grades of ductal elements or cordial structures that fill the stroma, papillary carcinomas are almost always well- circumscribed, soft, or moderately hard on macroscopic examination [12]. The most notorious histopathological feature of invasive carcinoma is the loss of myoepithelial cells. Although there are disagreements among authors, the incidence of myoepithelial cells can reach 10%. Although different histopathological evaluations, such as the amount of stroma in the papillary stalk and the nucleoplasm ratio in the epithelial component, are used to diagnose papillary breast cancer, there is still no definitive consensus on any of them, and dispute about this issue continues [13, 14].
As can be seen, it is extremely hard to diagnose invasive ductal cancer and papillary cancer properly; even histologically, it is not shocking that there are difficulties in diagnosing these diseases with non-invasive radiological methods such as ultrasonography and mammography. Furthermore, neither mammography nor ultrasonography has precise criteria to distinguish between invasive ductal cancer and papillary cancer [15]. Therefore, the authors recommend using both methods together against the possibility that these 2 radiological methods may fail to diagnose cancer [16].
Six basic morphological features have been defined for solid masses in Breast Imaging Reporting and Data System (BI-RADS) ultrasonography: shape, placement, edge, border, echo pattern, and back acoustic changes. A non-round or oval mass is described as an irregularly shaped mass. Irregularly shaped masses have a 60–100% risk of malignancy and must be evaluated with biopsy [17]. While the co-occurrence of oval shape and sharp edge suggests a benign process, the blunt edge is a feature that requires biopsy [17]. In addition, a thin, echogenic continuous, smooth pseudo capsule may indicate a slow-growing non-infiltrative lesion [18].
This study aimed to investigate and compare the ultrasonographic and mammographic findings of papillary breast carcinoma and invasive ductal carcinoma by considering all this literature. We identified some crucial findings in the results that we obtained. First of all, there was a significant statical difference between papillary cancer and invasive ductal cancer in terms of mammography contour irregularity in paired comparisons. Accordingly, while contour irregularity was detected in all invasive ductal cancer patients, only 77% of papillary cancer patients had irregularities. Similarly, the spicular pattern was detected in 95% of patients with invasive ductal cancer, and the spicular pattern was only seen in 59% of patients with papillary cancer. Our findings were similar to the studies reported in the literature. In a study conducted by Cha et al. (arch.) on 141 patients in 2018, one of the most common invasive ductal carcinoma mass patterns in ultrasonography was tumour contour irregularity [19]. Another study in 2015 conducted by Yang et al. found that one of the most common ultrasonographic findings in cases with invasive ductal carcinoma is contour irregularity (especially spicular irregularity) [20]. Also, in the same year, Jin et al. reported that contour irregularity on mammography was more prevalent among patients with invasive ductal carcinoma [21]. In another study published a few years before these studies, Janković et al. evaluated the mammography findings of 40 female patients. They reported that the spicular appearance was the most common [22].
As is commonly known, invasive ductal carcinoma is a type of breast cancer with a worse prognosis, and papillary breast cancer has a better prognosis; hypoechoic solid lesions with irregular-lobule contours on U/S and mammography or lesions with heterogeneous echoes with irregular contours are indicative of malignancy-risk [23]. Apart from these findings, another intriguing result that we obtained from our study was that the shape irregularity in U/S was statistically significantly higher in invasive ductal carcinoma than in papillary breast carcinoma, according to the rate of the lobulated tumour. U/S detected tumour shape irregularity in approximately 60% of patients with papillary cancer, and the remaining 40% of the tumour shape was lobulated. Our study detected 88% tumour shape irregularity and 12% lobulated tumour appearance in invasive ductal carcinoma. These results were consistent with the literature data and aligned with our investigations. On the one hand, Costantini et al. evaluated the ultrasonographic findings in 187 patients with breast cancer; they found that the irregular appearance of U/S was a significant indicator of malignancy for all types of invasive carcinoma, including invasive ductal carcinoma in BI-RADS stage of 3, 4, and 5 patients [24]. On the other hand, Stavros et al. found in malignant masses that the intraductal spread of the tumour is a finding that reflects the tissue transformation of the lobules to cancer or the growth of the tumour as finger-like protrusions [18].
Another interesting finding from our study was that the posterior acoustic changes in U/S were different in invasive ductal carcinoma and papillary carcinoma of the breast, while posterior acoustic shadowing accounted for 43% of all cases in invasive ductal carcinoma and acoustic flare was 4.5%. These rates were 13% for acoustic shadowing in patients with papillary carcinoma and 25% for acoustic flare in the remaining papillary carcinoma patients. According to these data, acoustic shadowing is a marker for invasive ductal carcinoma, and acoustic flare is a marker for papillary carcinoma. Our findings in this study were similar to the studies reported in the literature. Posterior acoustic shadowing is due to the weakening of the vocal bundle, and the presence of this finding is primarily considered in favour of malignancy. It has even been reported to have a positive predictive value of approximately 65–80% for malignancy [24, 25]. In the case of malignancy, shadowing is caused by the desmoplastic reaction that develops in the surrounding tissue rather than the mass itself. Acoustic shadowing is common in invasive ductal carcinomas that grow slowly; thus, it allows a desmoplastic reaction [20, 24]. Our findings were in line with those reported results in the literature.
Lastly, another intriguing finding that we detected in the binary statistical analysis was that homogeneity in internal tumour structures was detected in 75% of patients with papillary cancer. In comparison, heterogeneity was detected in 77% of patients with invasive ductal cancer by U/S examination. Throughout our search in the literature, it was reported that malignancy is seen less frequently in lesions showing homogeneity in ultrasonographic examinations. However, as heterogeneity increases, the probability of malignancy development also increases [26]. Our findings show similarity with the findings reported in the literature, especially considering the more aggressive course of invasive ductal carcinoma compared to papillary breast cancer.
The results that we discussed in other paragraphs in the discussion section were the results that we obtained from pairwise comparisons. Logistic regression analysis was performed to differentiate independent factors, invasive ductal carcinoma and papillary breast cancer, to determine which of the dependent variables in the binary statistical analyses were significant in differentiating these 2 entities from each other. Logistic regression analysis showed that only the ultrasonographic mass internal structure and ultrasonographic posterior acoustic shadowing had real effects that differentiated invasive ductal carcinoma from papillary carcinoma from the mammography and ultrasonography data. In other words, only acoustic shadowing on U/S and homogeneity in the mass internal structure for these 2 diagnostic methods were found to be significantly different between invasive ductal carcinoma and papillary carcinoma, i.e. only acoustic shadowing on U/S and homogeneity in the mass internal structure were significantly different between invasive ductal carcinoma and papillary carcinoma for these 2 diagnostic methods. It was seen that these 2 U/S data together had a correct diagnosis rate by distinguishing both types of malignancies in approximately 76% of the patients in the ROC analysis of these ultrasonographic data, which was performed to evaluate the success and performance of classifying patients in the diagnostic sense.
Conclusions
It is challenging to distinguish between invasive ductal carcinoma and papillary carcinoma of the breast, both ultrasonographically and mammographically, without a histopathological diagnosis. Neither ultrasonographic evaluation nor mammographic data are not enough to distinguish them from each other. Our study’s results favour invasive ductal carcinoma in terms of the increase in acoustic shadowing from ultrasonographic findings and the loss of homogeneity in the internal structure of the mass. Though the ultrasonographic and mammographic findings of invasive ductal carcinoma and papillary carcinoma are similar, it is still impossible to distinguish between the 2 types of cancer based on these 2 criteria alone. Thus, further studies are needed to distinguish these 2 types of cancer by non-invasive radiological methods, even without histopathological examinations. Also, there is a dire need for prospective studies with many patients, including more detailed and new methods other than classical ultrasonography and mammography.








