Introduction
The term density represents an attenuation of X-rays at the time they pass through fibro-glandular tissue. The fact that high breast density correlates with high rates of breast cancer is evident now. The prompt diagnosis of breast cancer in women with dense breast is a big challenge for modern radiology. It is generally known that mammography is only partially effective in solving this problem [1]. One of the last studies has identified advantages of breast tomosynthesis compared to full-filled digital mammography for women with dense breasts: the sensitivity for tomosynthesis was found to be significantly higher, reaching 77.4%, but the specificity was almost equal [2]. Contrast-enhanced spectral mammography (CESM) has been identified as a valuable modality in the differential diagnosis of breast lesions [3–7]. The sensitivity of CESM for diagnosing breast cancer has been reported between 90.5% and 100% [3, 8–11]. However, its specificity in discriminating malignant from benign lesions is highly varied and has been reported to be in the range 67.9–87.8% [8, 12–14]. Another study on the clinical performance of CESM in women with breast cancer in dense breast proved that CESM might be comparable to magnetic resonance imaging (MRI) in terms of radiological measurements of mass lesions in dense breasts, and moreover CESM showed less overestimated results than MRI – 16.2% for CESM and 22.7% for MRI [15]. Lesions visible in CESM include enhancing masses, non-mass enhancing lesions, and enhancing foci. No specific BI-RADS lexicon for CESM exists. CESM interpretation consists of the BI-RADS assessment based on low-energy (LE) images, which are equal to mammogram and assessment of BI-RADS descriptors for breast MRI. However, this lexicon is based on the evaluation of morphological characteristics of postcontrast enhancement of lesions and has no kinetic curve. Moreover, according to some articles [16, 17], final scoring is subjective. Thus, the final conclusion of CESM consists of the assessment of morphological characteristics of lesions on LE and recombined image (RI) combined. Although the establishment of the kinetic curve may provide new opportunities to maximize CESM accuracy, a literature review shows that there have been only 2 studies with different approaches for this issue [18, 19]. The purpose of this study was to analyse diagnostic performance of CESM based on morphologic and enhancement patterns of mass lesions in dense breast using different protocols: CESM without and with delayed image.
Material and methods
This retrospective study was approved by the local ethics committee. All enrolled patients provided written informed consent in this study.
Participant inclusion criteria: 1) mass lesions suspicious for malignancy (with and without microcalcifications) identified by mammography, ultrasound (US), or both; and 2) heterogeneously dense or extremely dense breast tissue. Participant exclusion criteria were as follows: 1) breast cancer confirmed by nearly done biopsy; 2) breast cancer history; 3) women with breast implant; 4) renal function impairment; 5) pregnancy or lactation; 6) allergic reaction to contrast agent; and 7) hyperthyroidism. Renal function impairment was evaluated by serum creatinine and glomerular filtration rate.
A total of 184 female patients aged 24–78 years (mean 46.9 ± 10.9 years) were included in the study during the period from September 2018 to April 2020. All of them had lesions that were suspicious for malignancy or inconclusive results by mammography and US. For the study all of them underwent CESM, which was performed by digital mammograph GE Senographe Essential Full Field Digital System (Mammography X-ray Equipment, Buc, France) using special software. After CESM 28 women with signs of benign lesions on CESM refused biopsy and started 2-year follow-up. They were excluded from the study. Also, there were 5 participants who had a light allergic reaction during CESM and did not finish the examination. They were also excluded. As a result, only 151 women were included in the study.
Contrast-enhanced spectral mammography technique
For fertile and premenopausal patients, CESM was conducted during the second week of their menstrual cycle, whenever possible; for postmenopausal patients there were no special requirements.
At the time of the study, a trained technologist obtained peripheral intravenous access in the antecubital fossa preferably with a 20-gauge needle. An iodinated contrast material (1.5 mL/kg dose) was administered intravenously. The catheter remained in place until the end of the examination.
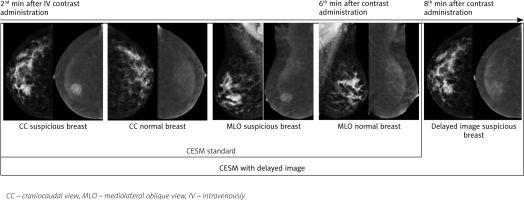
Image acquisition began on the 2nd minute after the beginning of the injection. The examination started with the craniocaudal (CC) view of the breast suspected of malignancy and was followed by the same view of the contralateral breast. Then, mediolateral oblique (MLO) views of both breasts were acquired in the same sequence and completed within 6 minutes. After that, a delayed image of the breast suspected of malignancy was presented only in CC view in order to avoid distortions caused by superimposition effect in MLO views (Fig. 1). As a result, we obtained delayed bilateral (lesions of both breasts) or monolateral (lesions of one breast) images in CC view 6–8 minutes after the initiation of contrast administration. The entire session took 8–10 minutes per patient, including contrast administration.
Low-energy images were performed at the same peak kilovoltage (kVp) and with the same filtration as FFDM, 26–30 kVp. High-energy acquisition was performed with a higher kVp of 45–49. RI were produced by the immediate cancellation of background breast tissue. Only the LE and RE images were sent to the archiving and communication system for interpretation.
After image acquisition CESM without delayed image and CESM with delayed image were blindly reviewed by 2 dedicated, sub-specialized, expert radiologists with more than 5 and 15 years of experience. The assessment of morphologic and enhancement patterns was based on the mammography and MRI lexicon version 5 by the American College of Radiology. On CESM without delayed image: all enhanced lesions considered to be malignant. To analyse CESM with delayed image we used a region of interest (ROI) indicator that was manually placed over the most homogenous area of lesion on RI: CC views on the 2nd and 8th minutes. The diameter of ROI was 2 mm. Based on mean signal in the lesion on RI: CC views on the 2nd and 8th minutes, we distinguished 3 types of contrast accumulation, similar to MR-curve types: 1) persistent – in the case of increasing the mean signal in the lesion by more than 10 units; 2) plateau – in the case of changing the mean signal in the lesion by less than 10 units; and 3) washout – in the case of decreasing the mean signal in the lesion by more than 10 units. Mass lesions with persistent type of kinetic curve were considered to be benign, while those with plateau and washout were considered to be malignant (Fig. 2). Finally, all participants were assigned a BI-RADS-category score of 1–5 for CESM without delayed image and CESM with delayed image.
Histological analysis
All patients underwent a biopsy and analysis of post-biopsied material in the Department of Pathomorphology. A total of 142 (91.6%) mass lesions were evaluated by US-guided biopsy; stereotactic biopsy was conducted for 7 (4.5%) mass lesions that were invisible on US; and postoperative material was analysed in 6 (3.9%) cases. Histopathological diagnostics were strictly provided after CESM in order to minimize contrast enhancement in the post-biopsied area and to obtain an accurate assessment of the enhancement type, which is especially crucial for masses that are less than 1.0 cm in size.
Statistical analysis
All data were analysed using SPSS ver. 27 software for Windows developed by StatSoft Inc. The χ2 test was applied to assess statistical differences between categorical patterns among malignant and benign lesions. The cross tabulations in dichotomized patterns were used to calculate accuracy, sensitivity, specificity, positive predictive value, and negative predictive value. P-values < 0.05 were considered statistically significant.
Results
Out of 151 females, 102 (67.5%) had heterogeneously dense and 49 (32.5%) had extremely dense breast tissue; 104 (68.9%) patients had mild breast parenchymal enhancement; 104 (67.7%) lesions were located in the upper-outer quadrant (Table 1).
Table 1
Patient characteristics
The quantity of lesions was 155, because 4 women had bilateral process. Among 155 lesions, 111 (71.6%) enhanced the contrast agent. Eighty-nine (57.4%) of them were histologically confirmed with malignancy, but 22 (14.2%) were benign. Forty-four (28.4%) lesions did not have contrast enhancement and were also pathomorphologically confirmed to be benign; hence, the absolute number of benign lesions was 66 (42.6%). All malignant lesions were performed with mass lesions and masses with calcification, 34 women were diagnosed with multifocal/multicentric processes. The distribution of malignant and benign lesions is shown in Table 2.
Table 2
Pathomorphological analysis of 155 lesions in 151 patients
Tables 3 and 4 demonstrate morphological patterns in LE images and RI (respectively) in relation to the pathomorphological analysis. The lesion shape pattern on LE images was found to be non-significant (p = 0.057), unlike the lesion margin pattern, which was found to be highly significant (p = 0.011). RI led to improvement of the significance of shape and margins, amounting to p = 0.002 and p < 0.001, respectively. Irregular shape, and irregular and spiculated margins were presented mostly in malignant lesions.
Table 3
Distribution of low-energy image patterns by histopathological results
Table 4
Distribution of recombined image patterns by histopathological results
The dynamic indicators of CESM with delayed image were determined to be highly significant. All 89 (100%) malignant lesions had enhancement on RI, and 44/66 (66.6%) benign lesions had no enhancement and were considered to be truly negative lesions (p < 0.001). The correlation between kinetic curve and histological results demonstrated that a persistent type of curve was common for benign lesions, accounting for 15/22 (68.1%); plateau and washout patterns were common for malignant lesions, accounting for 24/89 (26.9%) and 61/89 (68.5%), respectively.
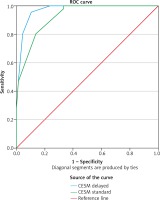
Table 5 compares diagnostic performance characteristics in CESM without and CESM with delayed image. Delayed image leads to an increase in specificity of up to 12.4%, which is statistically significant. The area under the curve (AUC) of ROC in CESM with delayed image is larger than that in CESM without delayed image (p < 0.01) (Fig. 3).
Table 5
Diagnostic performance characteristics of contrast-enhanced spectral mammography without delayed image and contrast-enhanced spectral mammography with delayed image
| CESM standard | CESM with delayed image | |
|---|---|---|
| Sensitivity (%)a | 97.8 | 93.7 |
| Specificity (%)a | 80.0 | 92.4 |
| PPV (%)a | 66.7 | 89.4 |
| NPV (%)a | 98.9 | 95.5 |
| Accuracy (%)a | 64.27 | 92.9 |
| AUCb | 0.924 (0.883–0.964) | 0.969 (0.942–0.995) |
| p-value | < 0.001 | |
Fig. 3
Receiver operating characteristic (ROC) curve for contrast-enhanced spectral mammography (CESM) with delayed image (blue line) and CESM standard (green line)
The dynamic kinetic curve analysis (Table 4) of 22 benign lesions detected a persistent type of curve in 15 (68.2%) lesions, plateau in 6 (27.3%) lesions, and washout in 1 (4.5%) case. There were 4 (2.6%) false negatives among 155 lesions, which had persistent curve type (sensitivity 93.7%) (Table 5). All of them were ductal carcinoma in situ (DCIS). Three of the remaining DCIS were invisible in LE images but enhanced contrast on RI, and 2 were visible on LE images but enhanced contrast only on delayed images. There were 7 (4.5%) false positive assessments on CESM with delayed image, 6 of them had plateau and 1 had washout (specificity 92.4%). A total of 3 cases of Phyllodes tumour and 3 fibroadenomas were related to plateau. The case of washout was confirmed with mastitis. The accuracy rate was 92.4% (Table 5).
Discussion
In view of the fact that density is accepted as one of the most important breast cancer predictors, breast cancer detection in the female Asian population with dense breasts is becoming the most critical issue for radiologists [20]. Mammography is known to be particularly effective for this purpose [1]. Ultrasound is good as a supplementary option to detail lesion features detected by mammography [21–23]. Magnetic resonance imaging of the breast is known as a gold standard of breast cancer detection due to its ability to reveal pathological neovascularity, which is historically associated with malignancy [21, 24]. However, it is apparent that benign lesions often enhance, causing insufficient specificity [25, 26]. The other challenge for using MRI is local inaccessibility and high cost [27, 28]. CESM is fundamentally identical to breast MRI in principle, and it has been reported to have a high rate of sensitivity but medium rate of specificity in the literature [3, 29, 30], which is caused by masking effects of the overlap of dense breast tissue. Hence it creates a demand for the development of new applications of existing methods.
Considering the ease of access and cost-effectiveness of CESM, we devoted this study to analysing the diagnostic performance of CESM based on morphological and enhancement patterns of mass lesions in dense breasts. We compared CESM patterns acquired by different protocols: CESM without and CESM with delayed images.
The goal of this study was to approach the quality of breast MRI. Thus, we integrated delayed images up to the 8th minute after contrast agent administration to acquire the kinetic curve based on the mean signal difference at the 2nd minute and 8th minute after the injection.
Using the ROC analysis, we determined that CESM with delayed image leads to improvement of specificity (AUC greater than 0.3). The persistent type of curve was typical for benign lesions, while malignant ones had plateau and washout.
The limitation of this study is the fact that we included only masses and did not analyse architectural distortion, asymmetry, and calcifications. Another limitation is that within the study, we discussed the likelihood of malignancy among mass lesions based on the contrast agent enhancement type. In the future, it would be reasonable to integrate the morphological and dynamic criteria to assess final category BI-RADS and explore disparities between breast MRI and CESM in malignant lesions.
Conclusions
CESM is sensitive for the differential diagnosis of breast lesions. CESM with delayed image has higher specificity than CESM without delayed image. Delayed images with plateau and washout are typical for malignancy. The assessment of mass morphologic features in combination with dynamic kinetic patterns of contrast enhancement will lead to significant improvement in the accuracy of CESM.