Summary
Computed tomography angiography (CTA) is as precise in prediction of coronary revascularization as invasive coronary angiography (ICA). This may add to the discussion about CTA having the potential to replace ICA for diagnosing vessels qualified for intervention, reserving the invasive diagnostic approach for those with the highest probability of revascularization.
Introduction
Coronary computed tomography angiography (CTA) has high diagnostic accuracy in ruling out significant stenosis of coronary arteries in patients with intermediate probability of coronary artery disease (CAD) [1–3]. Moreover, provided good image quality, CTA may also accurately assess previously implanted coronary stents suspected of in-stent restenosis [4, 5]. By definition, some of the patients examined by CTA have diseased coronary arteries and undergo further diagnostic testing, including invasive coronary angiography (ICA), and may undergo revascularization, either by percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Percent diameter stenosis (%DS) assessed by ICA plays a major role in clinical decision-making regarding coronary revascularization, as reflected in current European Society of Cardiology revascularization guidelines and clinical practice [6]. Importantly, recent studies suggest an increasing role of coronary CTA in evaluation of patients with higher (> 50%) pre-test probability of CAD, or blatant indications for invasive angiography [7, 8]. The evolving clinical landscape for use of CTA calls for better recognition of its value in clinical decision making as compared to the gold standard of ICA.
Aim
The objective of the present study was to assess the diagnostic value of quantitative coronary computed tomography angiography (QCT) as compared to quantitative coronary angiography (QCA) for the prediction of coronary revascularization.
Material and methods
Study group
From 09.2015 to 08.2016 we included 116 consecutive patients who underwent ICA following CTA performed at a single center in Warsaw, Poland. ICA was performed if CTA findings suggested significant or borderline coronary artery stenosis (> 50% DS, evaluated visually by experienced observer) in an artery amenable to intervention (at least 2.0 mm reference diameter), in the presence of clinical symptoms suggestive of CAD or additional tests indicating cardiac ischemia. Excluded from this group were patients (n = 10) who underwent ICA more than 6 months after CTA and those (n = 6) in whom CTA image quality prevented evaluation of the coronary artery lumen due to motion artifacts or severe calcification. Clinical and demographic information, medical history, and cardiovascular risk factors (hypertension, hyperlipidemia, diabetes, body mass index, smoking, being male) were prospectively collected. The local ethics committee approved this study.
CTA examination and analysis
Coronary CTA was performed on a dual source 2 × 192-slice Somatom Force (Siemens, Forchheim, Germany) scanner. Sublingual nitrates were administered prior to scanning in all patients. If necessary, β-blockers were administered intravenously targeting a heart rate < 70 beats per minute. The protocol for CTA image acquisition was recommended to comply with the Society of Cardiovascular Computed Tomography (SCCT) guidelines [9]. Assessment of luminal diameter stenosis was performed using an 18-segment coronary model. Quantitative diameter stenosis analysis (QCT) was performed with Syngo.via (Siemens Medical Systems) software by an experienced investigator blinded to the results of ICA. The intraobserver correlation coefficient performed in 60 randomly chosen vessels was 0.99 (95% CI: 0.98–0.99, p < 0.0001 for correlation). Per-vessel maximum stenosis was categorized as 0%, 1–24%, 25–49%, 50–69%, 70–99%, 100% according to SCCT guidelines [10]. Calcium score was calculated according to the Agatston method. Additionally, lesions were divided into non-calcified (no calcification), mixed (some calcification) and calcified (massive calcification) based on visual assessment.
ICA examination and angiographic analysis
The ICAs were performed on a standard cardiology fluoroscopy equipment (Axiom, Siemens Healthcare, Forchheim, Germany), in pulsed fluoroscopy mode with a default frame rate of 10 frames per second. Access site and utilization of additional tools (i.e. fractional flow reserve (FFR) assessment or intravascular ultrasound (IVUS)) was left to the discretion of the operator. Therapy decision was made on the basis of angiographic results in the context of the patient’s symptoms and other test results, such as stress ECG or echocardiography. Data from the literature suggest that visual vessel assessment during ICA is highly subjective [11, 12], so quantitatve coronary angiography (QCA) was chosen to define %DS in a repetitive manner. ICA images were submitted to Qangio XA (Medis, Leiden, The Netherlands) software for QCA analysis. Maximum diameter stenosis was automatically defined with subsequent manual alignment of the course of the vessel, if necessary. Per-vessel maximum stenosis was categorized as 0%, 1–24%, 25–49%, 50–69%, 70–99%, 100%. The analyses were performed for the right coronary artery (RCA), left main (LM), left anterior descending (LAD) and circumflex branch (Cx).
Statistical analysis
The categorical variables are presented as numbers and percentages. The continuous variables are expressed as mean ± SD or median (25th–75th percentile) as appropriate. Descriptive statistics were used to analyze per-vessel accuracy of CTA and QCA. The diagnostic performance of CTA and QCA in the prediction of revascularization was presented as accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) parameters and their corresponding 95% confidence intervals (CIs), as well as by receiver operator characteristics (ROC) analysis comparison. Paired samples t-tests or Wilcoxon test (as appropriate) were used to assess the equality of means in continuous variables. QCA and QCT comparison, including assessing lesions according to their calcification, was performed with the Bland-Altman test. A probability value of 0.05 or less was considered significant. All statistical analyses were conducted using MedCalc version 13.0 (MedCalc Software, Mariakerke, Belgium).
Results
In this study, 116 consecutive patients who underwent CTA and subsequently ICA were identified. After applying exclusion criteria, the final population consisted of 100 patients in whom 400 vessels (RCA, LM, LAD, Cx) were assessed. Baseline characteristics of the population (mean age: 67.1 ± 8.8, female 33%) are summarized in Table I. The majority of patients (54%) presented with typical angina. In this group Canadian Cardiovascular Society grade 2 was most commonly observed. Thirty-nine patients had undergone stress tests before coronary imaging – 24 tests had been positive electrocardiographically, 6 clinically, 3 had been inconclusive and 6 negative. Among 17 patients with PCI history, all stents were imaged by CTA without significant blooming artifacts precluding stent patency evaluation. During ICA, either FFR (n = 11, mean result 0.82) or IVUS (n = 5, mean MLA 5 mm2) was used in 16 cases. Mean time interval between CTA and ICA was 60.3 ±50 days. No serious adverse events were observed during either CTA or ICA.
Table I
Baseline patients’ characteristics and clinical assessment (n = 100)
| Parameter | Result |
|---|---|
| Age, mean ± SD [years] | 67.1 ±8.8 |
| Male gender, n = % | 67 |
| Height, mean ± SD [m] | 1.70 ±0.09 |
| Body weight, mean ± SD [kg] | 82.1 ±13.2 |
| Body mass index, mean ± SD [kg/m2] | 28.5 ±4.15 |
| Hypertension, n = % | 91 |
| Diabetes mellitus, n = % | 32 |
| Hyperlipidemia, n = % | 89 |
| Smoking history, n = % | 68 |
| Pack-years, mean ± SD [years] | 17.8 ±20.0 |
| Current smoker, n = % | 15 |
| Ejection fraction, mean ± SD (%)* | 61.1 ±8.1 |
| Atypical angina, n = % | 46 |
| Typical angina, n = % | 54 |
| CCS 1 | 7/54 (13.0) |
| CCS 2 | 28/54 (51.9) |
| CCS 3 | 16/54 (29.6) |
| CCS 4 | 3/54 (5.6) |
| Chronic kidney disease, n = % | 35 |
| PCI history, n = % | 17 |
| CABG history, n = % | 6 |
| AMI history, n = % | 13 |
| Family history of CAD, n = % | 28 |
| Stress electrocardiograph: | |
| Performed, n = % | 39 |
| Clinically positive, n (%) | 6/39 (15.4) |
| ECG-positive, n (%) | 24/39 (61.5) |
| Negative, n (%) | 6/39 (15.4) |
| Inconclusive, n (%) | 3/39 (7.7) |
| Serum total cholesterol, mean ± SD [mmol/l]: | 4.3 ±1.1 |
| Low-density lipoprotein, mean ± SD [mmol/l] | 2.5 ±0.9 |
| High-density lipoprotein, mean ± SD [mmol/l] | 1.4 ±0.4 |
| Statin, n = % | 90 |
| ACE-inhibitor or ARB, n = % | 86 |
| Calcium channel blocker, n = % | 44 |
| β-Blockade, n = % | 85 |
| Acetylsalicylic acid use, n = % | 100 |
Based on ICA findings PCI was performed in 53 patients, and 11 patients underwent subsequent coronary artery bypass grafting (CABG). Ultimately, 80 vessels were revascularized.
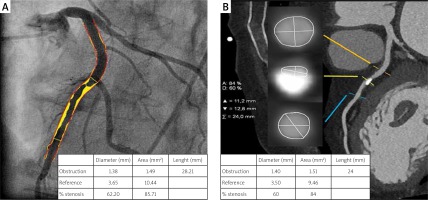
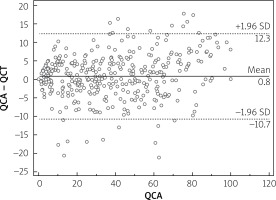
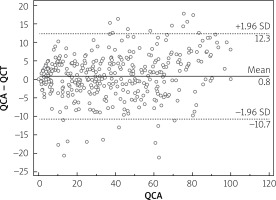
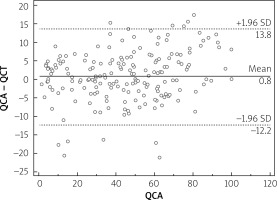
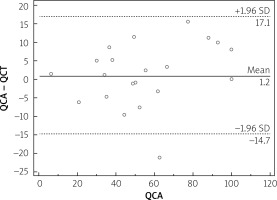
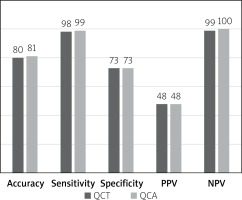
Median calcium score per patient was 424 (95% CI: 296–594). Mean stenoses of RCA, LM, LAD and Cx as assessed by QCA were 52%, 12%, 56% and 40%, respectively, and by QCT 50%, 11%, 59% and 40%, respectively. Per-vessel diagnostic accuracy, sensitivity, specificity, PPV an NPV were 80%, 98%, 73%, 48% and 99% for QCT and 81%, 99%, 73%, 48% and 100% for QCA, respectively, for prediction of revascularization (Figure 1). An example of QCT and QCA lesion analysis is presented in Figure 2. Bland-Altman plots assessing agreement between the two methods in all lesions, in non-calcified lesions, in mixed lesions and in stents are presented in Figures 3–6, respectively. AUC for predicting revascularization was similar: 0.88 for QCT and 0.89 for QCA (p = NS). No significant differences were found on an individual vessel level: AUC for the left anterior descending artery 0.79 and 0.79, for the circumflex artery 0.89 and 0.89, for the right coronary artery 0.84 and 0.85, for QCT and QCA respectively. Both methods performed similarly regardless of lesion calcification – AUC in non-calcified lesions (n = 193) for QCA was 0.957 (95% CI: 0.918–0.981) and for QCT 0.955 (95% CI: 0.916–0.980), whereas in mixed lesions (n = 201) AUC for QCA was 0.833 (95% CI: 0.774–0.881) and for QCT 0.825 (95% CI: 0.765–0.875). The reduction in AUC in mixed lesions compared to non-calcified lesions was statistically significant for both methods (p < 0.001 for both). Calcified lesions were too infrequent to warrant valid statistical analysis (n = 7). Considering previously implanted stents’ assessment (n = 23), further reduction in AUC (although not statistically significant as compared to mixed lesions) was observed, yet again comparable between the two methods – 0.676 (95% CI: 0.451–0.854) for QCA and 0.672 (95% CI: 0.447–0.851).
Figure 1
Per-vessel diagnostic performance of both cardiac CT scans and invasive angiography
Both tests demonstrated similar diagnostic accuracy for detecting vessels that eventually were revascularized. QCT – quantitative coronary computed tomography, PPV – positive predictive value, NPV – negative predictive value, QCA – quantitative coronary angiography.
Discussion
The CTA, as a non-invasive diagnostic method, is recommended for patients with intermediate CAD probability [13] and is widely used as first-line diagnostics in patients with suspected CAD. Recent studies suggest that the method can be effectively used in patients with direct indications for invasive angiography [7, 8]. The expanding diagnostic role of coronary CTA calls for better recognition of its capacity to provide the basis for clinical decision making. We found that the predictive accuracy of coronary CTA-derived measurements of stenosis severity had similar diagnostic accuracy compared with QCA in prediction of coronary revascularization, which was impacted neither by presence of calcifications nor by stents. This finding suggests that, due to lower costs and improved safety, coronary CTA may act as a gatekeeper and first-line test to triage patients to medical therapy or invasive evaluation with potential revascularization. The high specificity and NPV of CTA are comparable with previous studies [1, 2]. The relatively low PPV of both methods is expected and related to the fact that not all lesions with stenosis >50% in diameter necessitate intervention, as other factors (i.e. clinical symptoms or FFR measurement) influence the revascularization decision [14]. Our data are consistent with three papers from recent years, in which measurements derived from CTA performed similarly to QCA-derived measurements in assessing luminal stenosis, at both a per-patient and per-vessel level [15–17]. Our results are also in line with previously reported, slightly lower accuracy of CTA in lesions with calcifications [18] or previously implanted stents [4, 5]; however, in our dataset also QCA performed worse in such conditions. Furthermore, in the mentioned studies CTA findings accurately evaluated ischemia-causing lesions, with invasive FFR as a reference standard, which is accordant with our conclusion about CTA ability to predict coronary revascularization. Additionally, besides anatomical assessment provided by CTA, functional assessment became recently feasible with CTA-derived FFR. It emerged as a promising, non-invasive diagnostic tool, whose main advantage over plain coronary CTA is improved specificity for diagnosing functionally significant stenosis [19, 20].
Study limitations
We acknowledge several limitations of the current study. The sample size is limited and is derived from a single center. The study was conceived as an observational, non-randomized project, based on prospectively collected data. Non-randomization and the fact that several operators performed ICAs may have influenced procedural aspects such as use of additional tools (i.e. FFR, IVUS) and therefore may have, to some extent, affected revascularization decisions. This, however, reflects everyday practice.
Recognizing its inherent limitations, the study does have the advantage of representing the daily practice in a diverse patient population, as no clinical exclusion criteria were applied.
Conclusions
These real-world data support the concept that CTA is as precise in prediction of coronary revascularization as ICA. This may add to the discussion about CTA having the potential to replace ICA for diagnosing vessels qualified for intervention, reserving the invasive diagnostic approach for those with the highest probability of revascularization.