Despite the progress that has been made in the treatment of coronary heart disease, there is still a group of patients (approx. 2–3%) whose angina symptoms persist in spite of an optimal therapy (refractory angina, no-option angina). The concept of increasing blood flow to the ischaemic myocardium through revascularization procedures (percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)) is the gold standard of the treatment. The development of cardiosurgical methods and percutaneous recanalization techniques, as well as the extension of standard pharmacotherapy, allowed a further reduction of symptoms only in some patients. For those symptomatic patients a coronary sinus flow reducer may be a solution [1]. The essence of its action assumes that the obstruction of the blood outflow from the coronary sinus causes an increase in pressure in the venous part of the coronary circulation and in the microcirculation, and facilitates the delivery of oxygen to myocardial cells. An indication for an implantation of the reducer is refractory angina, defined as the persistence of symptoms for more than 3 months despite the combination of pharmacotherapy, angioplasty, and CABG (class II b). These criteria were met by a 70-year-old patient with obesity (BMI = 45.66 kg/m2), hypertension, hyperlipidaemia, type 2 diabetes, after a stroke, CABG, multiple PCI, and pacemaker implantation. The patient was made to use nitroglycerin up to 20 times a day in the period preceding the treatment. Due to the exhaustion of therapeutic options (no possibility of further revascularization of the coronary arteries), the patient was qualified for implantation of a sinus-coronary flow reducer.
Under local anaesthesia, the right internal jugular vein was punctured under ultrasound guidance, and a coronary sinus angiography was performed (Figure 1 A). After confirming favourable anatomical conditions, a dedicated 9F vascular sheath and guide catheter were inserted using an ultra-stiff guidewire. Thereafter, the ReducerTM expanded on the balloon was loaded, positioned, and implanted under the pressure of 6 atm (Figure 1 B). Control coronary sinus angiography revealed a properly expanded hourglass-shaped implant (Figure 1 C). The patient did not feel any significant clinical improvement for 4 weeks. Eight weeks after the procedure, there was a significant reduction in symptoms from CCS IV to CSS II class, which may be associated with complete device endothelialisation with controlled reduction of coronary sinus lumen to an expected 3 mm diameter after device implantation. The cardiac computed tomography (CT) scan performed in the eighth week after the implantation showed a correctly positioned implant in the coronary sinus (Figure 1 D).
Figure 1
A – Initial coronary sinus angiography. B – Implantation of Reducer under pressure of 6 atm. C – Hourglass-shaped implant in control coronary sinus angiography. D – The cardiac computed tomography (CT scan) in the eighth week after Reducer implantation
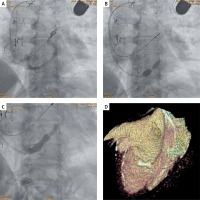
It is relevant for the ReducerTM implantation that the characteristic hourglass shape of the implant is not visible during the control angiography ending the procedure (Figure 1 C). The clinical effect after endothelialisation depends on the blood flow through the obtained controlled coronary sinus stenosis, similar in shape to what we see at the time of implantation, when filling the balloon with contrast (Figure 1 B).
The coronary sinus flow reducer is a valuable addition to the available therapeutic arsenal in the fight against coronary artery disease, especially for so-called “no-option” patients [2]. The number of ReducerTM implantation procedures has been growing rapidly in the last 2 years. Although the first reducer in our centre implanted in December 2020 was one of the first in Poland, it should be expected that the number of implantations will increase significantly if the procedure is reimbursed in a precise medical indication as “no-option” refractory angina. For this reason, it is beneficial if medical staff assessing all chest radiographs are able to identify the characteristic implant and the difficult patient behind this image.








