Introduction
Colorectal cancer and pancreatic ductal adenocarcinoma
Colorectal cancer (CRC) and pancreatic ductal adenocarcinoma (PDA) are two of the leading causes of death worldwide, placed as the second and fourth cancer-associated deaths in the USA, respectively [1–4].
Over 35% of colorectal cancers are hereditary or develop as a continuation of inherited susceptibility to this disease [1].
The chromosomal instability and physical loss of wild-type tumour suppressor genes such as p53 and SMAD4 are present in both colorectal and pancreatic cancers. Genetic mutations are also associated with gene receptors such as TGFBR2. CRC drug treatment programs are principally focused on the absence of KRAS/NRAS/BRAF mutations. In some colon tumours it has been verified that there is a presence of microsatellite instability (MSI).
In CRC the TGF-β tumour suppressor pathway changes and is connected with inactivation mutations of TGFBR2, which are present in one third of patients. Research has shown that no new mutations are required for metastasis [1].
Most patients diagnosed for PDA are in late stages of the disease, and only 10–15% of tumours are resectable because of late and difficult diagnoses. Inflammation and chronic pancreatitis are still mentioned as major risk factors for cancer. The tumour microenvironment is still changing, in particular, during progression to invasive PDA [5].
There is significant proof that both cancers could have a genetic basis. Despite environmental causes, including inflammation, there are many genes involved in carcinogenesis. In our previous paper, we mentioned possible genetic causes of pancreatic ductal adenocarcinoma [6].
Tumour necrosis factor α
Tumour necrosis factor (TNF) is a crucial element in immune surveillance and is also required for proper functioning and proliferation of many cells (NK, lymphocytes T and B, macrophages, dendritic cells) involved in immune response [7]. TNF-α exists as soluble and membrane forms and is involved in inflammation and autoimmunity. This cytokine is present in a chronic inflammatory condition, which is a known cause of carcinogenesis [8].
In a cancer microenvironment, natural functions of TNF are changed and are observed in all stages of tumorigenesis. It is particularly important during early tumour promotion stages. TNF-α becomes a tumour cell product, including in both colon carcinoma and pancreatic cancer. In the latter it mediates proliferation through induction of expression of EGFR and TGF-β and modulates autocrine growth-regulatory pathways. Observations suggest that tumour necrosis factor is responsible for promotion of cell migration through disruption of epithelial cell adhesion. The role of TNF-α in angiogenesis is still being investigated, but it is also suggested to have a role in vessel growth promotion in tumours [7].
In-vitro and animal models proved that tumour necrosis factor protein, in high concentrations, is capable of killing tumour cells. In invasive colon cancer models, it promotes an epithelial-mesenchymal transition, and when the factor is inhibited, it has a strong anti-tumour influencein pancreatic ductal adenocarcinoma [9].
Transforming growth factor β1
Transforming growth factor (TGF) and its family members are involved in many biological processes. One of the better-known functions of anti-inflammatory TGF-β cytokine is growth inhibition of many cell types. Despite the role of TGF-β in cell development and differentiation, it is also an important factor during wound healing and regulation of inflammatory processes. TGF-β inhibits proliferation and function of T- and B-lymphocytes, as well as proliferation of endothelial and epithelial cells. Factors regulate protein expression influencing inhibition of cell cycle activity. The most abundant form of this cytokine is TGF-β1.
Detection of increased levels of this cytokine in cancer patients might indicate either tumour suppression or progression. This is known as a TGF-β paradox. The potent suppressive role of TGF-β is reported in early stages of cancer, while in the latter stages it is associated with angiogenesis through VEGF expression and can also play a role as a pro-metastatic mediator. It is also known that tumour cells, via cytokine expression (e.g. TGF-β), can reduce inflammation and use immunosuppression to their advantage [10–13].
Our previous research on cytokine levels, in various types of inflammation, suggested that polymorphism of TNF-α and TGF-β genes can play a key role in soft tissue infections [14–16]. The aim of the present study was to investigate markers of bacterial infection, surgical wound healing disorders, and/or fistula anastomosis in patients undergoing surgery for pancreatic and colorectal cancer. For this reason, polymorphism of TGF-β (c.29C>T) and TNF-α (c.-308G>A) genes were examined by genotyping using TaqMan probes, and results were correlated with the level of the TNF-α and TGF-β in the blood serum. To our knowledge, this is the first paper focused on the association of TNF-α (c.-308G>A) and TGF-β (c.29C>T) polymorphisms and the serum levels of its proteins together. In most papers, cytokine levels or polymorphisms are treated separately with reference to a particular type of cancer.
Material and methods
Characteristics of the examined groups
The study group consisted of 65 patients (33 men, 31 women), who met the criteria for inclusion and exclusion of pancreatic cancer, and 41 patients (25 men, 16 women) with colorectal cancer. Patients included in the study were hospitalised in the Department of Gastroenterology and Transplantology of the Central Clinical Hospital, Ministry of Interior from 2011 to 2015. The occurrence of both types of tumour was confirmed by histopathological examinations performed in the Department of Pathomorphology of the Central Clinical Hospital, Ministry of Interior.
The patients were subjected to routine clinical evaluation, such as biochemical, bacteriological, and imaging studies. The final stage of the evaluation was the infection of wound coatings, anastomotic separation, abscess of the anastomosis, or sepsis. The exclusion criterion was stage IV according to TNM classification with complications such as jaundice, portal vein obstruction, intraperitoneal dissemination, and colonic ileus with indication for urgent surgery.
The control group consisted of 100 healthy volunteers (63 men, 37 women) without lesions in the respiratory, urinary, and gastrointestinal tracts (according to the personal medical history).
All patients’ data, excluding information required for including and excluding criteria, type of tumour, and sex, was kept fully confidential.
Ethics
The patients gave written consent to the details of the protocol, which were fully explained. The protocol of the study was approved by the Medical University Ethics Committee and conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki.
Determination methodology
The clinical specimens collected from each patient from both the examined group and the control group for genetic tests were full peripheral blood taken with EDTA anticoagulant and clot. Molecular tests were performed at the Department of Surgical Research and Transplantology at the Mossakowski Medical Research Centre Polish Academy of Sciences and included:
The study of gene polymorphism for TNF-α(c.-308G>A) and TGF-β (c.29C>T) by genotyping using TaqMan probes (Life Technologies)
To examine polymorphisms of the above genes, genomic DNA was isolated using the Nucleo Spin Blood kit (Macherey-Nagel, Germany) according to the manufacturer’s instructions. Concentration and purity of the isolated genetic material was measured using a Nano Drop ND-1000 spectrophotometer (Nanodrop Wilmington, USA). Gene polymorphism analyses were performed on a Step One Applied Biosystems device. In the case of TNF-α polymorphism (c.-308G>A), a probe for rs number 1800629 was used for analysis, and for TGF-β (c.29C>T) a probe for polymorphism of rs1800470 (previously rs1982073) was used. The thermal profile for the above probes included the following steps: enzyme activation (95°C for 10 minutes), denaturation (95°C for 15 seconds), and probe attachment (60°C for one minute). The last two stages were repeated 40 times. Genotypes were analysed using TaqMan Genotyper Software.
Determination of TNF-α and TGF-β protein concentration in blood serum by ELISA method
The concentration of TNF-α and TGF-β in serum in all the examined groups was determined with commercially available enzyme-linked immuno-sorbent assay (ELISA) kits, Tumour Necrosis Factor Alpha (TNF-α) from Cloud-Clone Corp. and Human TGF-β Immunoassay from the R&D System, according to the manufacturer’s instructions. The labelled immunoenzymatic reaction product was evaluated based on the spectrophotometric method at a wavelength of 450 nm. Sample determination was duplicated. The concentration of the examined proteins was evaluated by comparing the obtained absorbance values with the standard curve prepared by determining the absorbance of samples of known concentration. The concentrations were given in pg/ml.
Statistical analysis
SAS 9.4 was used for most of the analysis. The analysis of deviations from the Hardy-Weinberg equilibrium analysis was carried out using the program written ad hoc in Fortran, using IMSL libraries and the subroutine SNP-HWE by Wigginton et al. [17]. The participation of each genotype in the examined groups was presented in the form of percentages.
Correlations between qualitative traits (genotype-group) were determined by odds ratio (OR). The strength of the correlation was estimated by intervals, at a confidence level of 95%. The result was supplemented by the calculated level of statistical significance (p-value). The statistically significant parameters were those variables for which the significance level p was less than 0.05.
Correlations between qualitative and quantitative traits (group or genotype vs. protein level, including gender; genotype vs. protein level, including gender and group) were determined using the general linear model. Adjusted interval estimates for average effects were obtained from the least-squares method (least-squares means), at a confidence level of 95%. These results were supplemented by calculated levels of statistical significance (p-values).
Due to the strong positive skewness of the distributions, the protein levels were log-transformed before the analysis; final estimates (point and interval) were obtained by inverse transformation (antilogarithm finding).
Results
The prevalence of individual genotypes in patients with pancreatic cancer (n = 64), in patients with colorectal cancer (n = 41), and in the control group (n = 100) and their comparison are presented in Table 1.
Table 1
Prevalence of individual genotypes. M1 is the model of total dominance (WW + WM vs. MM) and M2 is the model of semi dominance (WW vs. WM + MM)
In the first stage of the study, the c.-308G>A polymorphism in the promoter sequence of the TNF-α gene was examined. In the case of patients with both pancreatic and colorectal cancer groups, there were no significant differences in the prevalence of genotypes GG and GA and the control group. The percentage distribution of the mutated AA homozygous genotype was different. In patients with colorectal cancer, it was much more frequent than in the second study group and control group (7.3% vs. 3.2% vs. 1.0%, respectively). However, the observed difference in the distribution of genotypes was statistically insignificant, p = NS.
Comparing genotypes of the c.29C>T polymorphism of the TGF-β gene between the group of patients with colorectal and pancreatic cancer, there were no significant differences in the presence of CC homozygous mutant genotypes compared to the control group.
The second part of the study concerned a comparison of the level of TNF-α and TGF-β protein between the patients with pancreatic and colorectal cancer, and the control group. The results are presented in the form of arithmetic means in Figures 1 and 2.
Fig. 1
Comparison of TNF-α protein concentration between patients with pancreatic and colorectal cancer and the control group
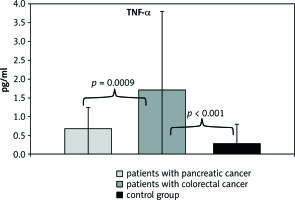
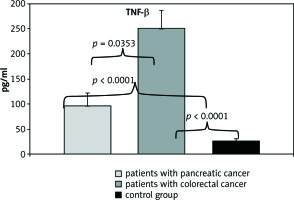
Fig. 2
Comparison of TGF-β protein concentration between patients with pancreatic and colorectal cancer and the control group
The mean TNF-α concentration in patients with colorectal cancer (1.72 ±2.10 µg/ml) was significantly higher compared to the control group (0.29 ±0.52 µg/ml, p< 0.0001). There was no statistically significant difference in TNF-α concentration in patients with pancreatic cancer (0.69 ±0.56 µg/ml) in relation to the control group, p = 0.0829. A statistically significant difference was noted when comparing both study groups, p = 0.0009.
In turn, TGF-β concentration in both study groups was significantly higher, at 97.06 ±24.75 µg/ml and 251.86 ±35.19 µg/ml, respectively, compared to the median concentration (26.45 ±3.87 ρg/ml) in the control group, p< 0.0001 in both cases. It was also noted that the concentration of TGF-β protein was significantly higher in patients with colorectal cancer than in patients in the second group. These results were also statistically significant, p = 0.0353.
The next stage of the study was an attempt to evaluate the effect of two polymorphisms, c.-308G>A of the TNF-α and c.29C>T TGF-β genes, on the value of their protein concentration. Results are illustrated in Figures 3–6.
Fig. 3
The concentration of TNF-α protein in patients with pancreatic cancer for particular genotypes
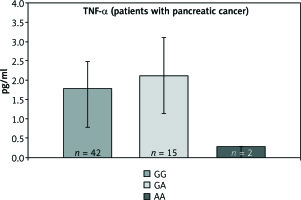
Fig. 4
The concentration of TNF-α protein in patients with colorectal cancer for particular genotypes
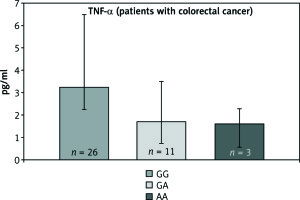
Fig. 5
The concentration of TGF-β protein in patients with pancreatic cancer for particular genotypes
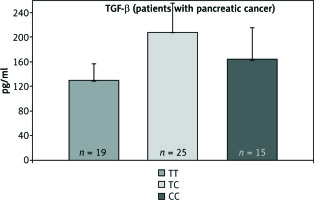
Fig. 6
The concentration of TGF-β protein in patients with colorectal cancer for particular genotypes
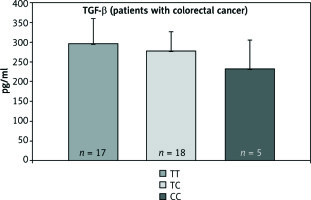
The analyses show that the occurrence of the polymorphic genotype -308AA of the TNF-α gene is not correlated with increased concentration of the examined protein in patients with both pancreatic and colorectal cancer.
In the case of c.29C>T polymorphism, it was also not observed that the presence of the polymorphic CC gene allele caused an increase in TGF-β protein concentration in both groups.
Statistical analysis, aimed at comparing the arithmetic mean of the concentration of tested proteins between the three genotypes, did not show statistically significant differences.
Discussion
The TNF-α and TGF-β factors, as pro- and anti-inflammatory cytokines, respectively, were described in earlier papers from our department in cancers and other pathologic conditions [14–16]. In our last paper [16], in which we compared genotypes in polymorphic sites in TNF-α and TGF-β genes with their expression levels, we showed that the presence of polymorphic alleles could predispose to increased production of their proteins. Patients with prolonged non-healing wounds should have their genotypes studied and in the case of mutation, long-term antibiotic and immune protein supply should be considered.
Many papers treat the role of TNF-α and TGF-β cytokines in pancreatic and colorectal tumours separately. In this paper we tried to compare the two polymorphisms, which influence the transcription of their proteins, as well as the circulation levels of both cytokines in patients’ serum in specific tumour conditions. Each patient in both groups of tumours was studied for both cytokines’ states (polymorphisms and circulating protein levels).
Despite the fact that the levels of both cytokines described in the tumours in this paper change depending on the stage of tumour development, we searched the possible association between particular allele state and the level of serum protein.
We did not study the stage of tumour development, but we are aware of the fact that elevated serum levels of these cytokines are an indicator of poor prognosis mostly connected with malignancies [18].
The polymorphism NM_000594.3 (TNF): c.-488G>A (rs1800629) is better known as a TNF-α-308 SNP. This polymorphic site is associated with autoimmune diseases and others in which macrophages and monocytes play a crucial role [17]. The A allele is associated with higher levels of TNF expression and occurs with a frequency of 13%. The most common G variant occurs with a frequency of 87% (1000 Genomes Project Phase 3 allele frequencies). Abraham et al. concluded that the G to A change indirectly affects transcription of TNF protein through the effect of binding capacity of other proteins, such as nuclear factors [8, 19, 20]. We noticed that in colorectal cancer the allele A was more frequent than in the pancreatic cancer group; however, we did not confirm its influence on increasing the serum level of its protein.
The polymorphism NM_000660.6 (TGFB1): c. 29C>T (p.Pro10Leu) rs1800470 (formerly rs1982073) is also identified as c.867C>T. The polymorphic site is characterised as a regulator of TGF-β protein expression, increasing the transcript level in plasma in the presence of the 29C allele [21]. In both studied groups we did not note a higher representation of allele C in comparison to the control group. As for the case of TNF-α polymorphism, we did not confirm an increased level of serum protein associated with the presence of allele C in both groups.
It seems, therefore, that the factors we examined in the peripheral blood and serum do not have a very strong effect on the cancer process taking place in a specific organ, nor do they have an effect modulating the level of the pro- and anti-inflammatory cytokines tested. Some influences cannot be excluded due to the group sizes being too small to make precise estimates. The main problem with small studies is interpretation of results, in particular confidence intervals and p-values. For this reason, the studied groups should be larger to get more reliable results. Additionally, the statistical tests normally require a larger sample size to ensure a representative distribution of the population and to be considered representative of groups of people to whom results will be generalised.
Conclusions
The results obtained allowed us to make the following conclusions:
The only statistically significant effects were the correlations between patients belonging to a specific group (pancreatic cancer/colorectal cancer/control) and average protein levels.
Despite numerous literature reports about more frequent cancer lesions in the case of male patients, our research cannot confirm or deny this data. We should take into account the small number of individual tests covering only patients operated due to the detection of a specific type of cancer over four years in one of the key surgical centres in Poland: the Central Clinical Hospital of the Ministry of the Interior.
Analysis of the frequency of genotypes for particular genes most often did not provide evidence of deviations from Hardy-Weinberg’s equilibrium. The exceptions for which the deviation is clear are TNF-α in the group of patients with colorectal cancer. The analyses included both a completely dominant model (WW + WM vs. MM) and a model of semi dominance (WW vs. WM + MM) where W meant the existence of wild-type allele and M mutant.
No effect of sex or genotype on the occurrence of elevated levels of TNF-α and TGF-β protein control was seen despite their variability in particular types of cancer. In view of the relatively small sample size and the resulting significant statistical uncertainties, it is not possible to exclude these types of effects.








