Introduction
The incidence of type 1 diabetes (T1D) has been growing worldwide, particularly in Europe [1]. In response to this trend, continuous subcutaneous insulin infusion (CSII) has become increasingly common in the treatment of T1D. This approach involves the use of both traditional personal insulin pumps and more advanced automated insulin delivery systems, which are currently reimbursed in Poland. CSII enables continuous insulin delivery tailored to the PwT1D’s needs, offering advantages over multiple daily injections [2]. Although the efficacy of CSII therapy has been demonstrated by ample research [2, 3], there have been no cross-sectional studies assessing current treatment outcomes in Polish adults, particularly in light of recent technological advances and transition from paediatric care [4]. The treatment of T1D requires daily commitment due to the need for self-administration of insulin. Therefore, diabetes education is a crucial component of treatment, and scientific societies emphasise the effectiveness of structured educational programs. Diabetes Poland recommends that therapeutic education be comprehensive, patient-centred, and evidence-based [5, 6]. For PwT1Ds treated with personal insulin pumps and continuous glucose monitoring (CGM) systems, education at initial stages should take at least 9–11 hours [7].
One of the main goals of T1D treatment is to achieve and maintain appropriate metabolic control to minimise the risk of neurological and vascular complications [9]. Metabolic control is assessed using parameters such as time in range (TIR) and/or glycated haemoglobin (HbA1c) levels [7, 8]. The reasons why metabolic targets are not achieved in people treated with personal insulin pumps are complex [10–12]. PwT1Ds often do not use all the features of the pump, or the pumps are not programmed correctly. Other reasons for poor treatment outcomes include inadequate education and psychological barriers, such as lack of acceptance of the disease and mental health issues [10]. There is growing awareness that education should be tailored to the treatment profile and the needs and abilities of the individual PwT1D. As a result, more structured training programs have been developed worldwide, such as INPUT (Insulin Pump Treatment), SUBITO (Insulin Pump Therapy for Adult Patients with Diabetes Mellitus Type 1) in Germany, and DAFNE (Dose Adjustment For Normal Eating) in the UK, or the re-education program at Dokuz Eylul University in Turkey. Evaluations of these initiatives have shown that education tailored to the PwT1D’s treatment profile improves HbA1c levels, lowers the risk of severe hypoglycaemia episodes, reduces glycaemic variability, and improves knowledge about treatment and advanced pump features (e.g. temporary basal rates, bolus options), thus reducing the risk of depression and diabetes-related stress, and improving the PwT1D’s quality of life [13–17].
In 2008, a proprietary educational program for PwT1Ds treated with personal insulin pumps was implemented at the Department of Internal Medicine and Diabetology at Poznan University of Medical Sciences in Poznan, Poland. The training program is offered to PwT1Ds during “Insulin Pump Week” hospitalisation for poorly controlled diabetes. It includes 9 hours of education in groups of 10 to 15 people treated with personal insulin pumps. The topics covered include T1D and the basics of CSII therapy. In 2023, the program was given a structured framework and multimedia support and was officially named the “GoPump Structured Diabetes Education Program” [18].
Aim
The aim of this cross-sectional study was to assess PwT1Ds treated with CSII who participated in the GoPump program and to identify factors associated with treatment effectiveness.
Material and methods
The study included individuals hospitalised at the Department of Internal Medicine and Diabetology at Poznan University of Medical Sciences during the “Insulin Pump Weeks” between 2022 and 2023. PwT1Ds who were referred from outpatient diabetes clinics and treated with intensive functional insulin therapy using CSII before hospitalisation were included in the study. Exclusion criteria were the lack of consent to participate in the study, new-onset diabetes, the use of automatic insulin delivery systems (AID) at the beginning of the study, hospitalisation for emergency conditions (including diabetic ketoacidosis), and pregnancy. PwT1D provided informed and voluntary consent to participate in the study, and the study was approved by the Bioethics Committee at Poznan University of Medical Sciences (no. 796/22).
Medical history, physical examination, and acute and chronic diabetes complications
PwT1Ds’ medical histories were reviewed to obtain data on the duration of diabetes, the duration of CSII treatment, the presence of chronic diabetes complications, and the occurrence of acute diabetes complications in the past 12 months. The subjective physical activity level was used to assess the intensity of daily physical activity, ranging from 1.2 (sedentary lifestyle) to 2.0 (extremely active) [19]. For individuals with a history of smoking, pack-years were calculated as the product of the number of packs of cigarettes smoked per day and the number of years of smoking. Body mass index (BMI) was calculated based on weight and height.
Data from personal insulin pump, blood glucose meters, and CGM reports
On hospital admission, data from each PwT1D’s device were retrieved and evaluated for the 28 days preceding hospitalisation. Insulin pump reports were used to assess total daily insulin (TDI) per kilogram of body weight (TDI/kg bw), the amount of insulin in the basal infusion, basal-to-bolus ratio, median number of boluses per day, and the percentage of boluses using the bolus calculator. The use of advanced pump features, such as dual-wave and square boluses as well as temporary basal rate, was assessed twice: 1) based on self-reports by PwT1Ds who completed a questionnaire on the day of hospital admission; and 2) based on reports from insulin pumps. For individuals using CGM systems, median glucose levels and glycaemic variability (coefficient of variation) were assessed, along with sensor usage time and time spent in different glycaemic ranges (> 250 mg/dl, 181–250 mg/dl, 70–180 mg/dl, 54–69 mg/dl, and < 54 mg/dl). For individuals using blood glucose meters, median glucose levels with standard deviation (SD), number of glucose measurements per day, and number of glucose readings below 70 mg/dl and below 54 mg/dl were assessed.
Laboratory tests
Laboratory tests included HbA1c and serum concentrations of total cholesterol (TC), triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and non-HDL cholesterol (non-HDL-C). The tests were performed using a Cobas 6000 device (Roche Diagnostic, Basel, Switzerland). The HbA1c value was determined using turbidimetry. Serum TC, triglycerides, and HDL-C levels were measured using the enzymatic calorimetric method. LDL-C levels were calculated using the Friedewald formula, and non-HDL-C levels were calculated based on TC and HDL-C measurements.
Diabetes Treatment Satisfaction Questionnaire
Satisfaction with diabetes care was assessed using the standardised Diabetes Treatment Satisfaction Questionnaire – Status Version (DTSQs), which evaluates 8 different aspects of treatment satisfaction, rated on a scale of 0 to 6, with a maximum score of 36 points. Questions regarding hypoglycaemia and hyperglycaemia were assessed individually and were not included in the total score. PwT1Ds reported their recent experience with unacceptably high or low blood glucose levels on a scale from 0 (never) to 6 (most of the time) [20].
Statistical analysis
Data processing and statistical analysis were performed using the R programming language (version 3.6.1, R Project, Vienna, Austria). We utilised the Shapiro-Wilk test to assess the normal distribution of the analysed continuous variables. Since nearly all the variables did not exhibit characteristics of a Gaussian distribution, we decided to employ non-parametric tests. First, descriptive statistics were analysed, and the results were expressed as the median (Q1–Q3: 25th–75th percentile). Then, the Spearman rank correlation test was performed to assess correlations between diabetes-related features and the use of CGM system or blood glucose meters.
Results
Medical history, physical examination, and acute and chronic diabetes complications
The general characteristics of the study group are presented in Table I. The study included 107 adults (65 women [60.7%]) with a median age of 26.7 years (Q1–Q3: 19.0–30.8) and a median T1D duration of 13 years (Q1–Q3: 10.0–18.0). The median BMI was 23.9 (Q1–Q3: 21.8–26.3) kg/m2, and the median physical activity level was 1.4 (Q1–Q3: 1.4–1.6). A history of smoking was reported in 33 individuals, of whom 21 (19.6%) were active smokers at the time of the survey. The presence of at least one chronic diabetes complication was reported in 11 PwT1Ds, including retinopathy in 10, peripheral neuropathy in 5, and diabetic kidney disease in 2. Six individuals (5.2%) reported at least one episode of diabetic ketoacidosis in the past 12 months. Six individuals (5.2%) had at least one episode of severe hypoglycaemia in the past 12 months, with 2 PwT1Ds reporting recurrent severe hypoglycaemia.
Table I
Characteristics of the study group (n = 107)
Data from personal insulin pump, blood glucose meters, and CGM reports
Data from personal insulin pumps, blood glucose meters, and CGM systems are presented in Table II. In the study group, 5 individuals (4.8%) used the YpsoPump, 20 individuals (18.7%) used the Accu-Chek Combo pump, and the remaining 82 individuals (76.6%) used Medtronic pumps. Insulin aspart was used by 52 individuals (48.6%), lispro by 38 (35.5%), faster aspart by 14 (13.1%), glulisine by 4 (3.7%), and human insulin by one individual (0.9%). The median duration of pump therapy was 8.0 years (5.0–12.0). TDI/kg bw, total daily insulin dose, basal infusion rate, basal-to-bolus ratio, the median number of boluses per day, and the percentage of all boluses administered using the bolus calculator are presented in Table II.
Table II
Data from personal insulin pumps, blood glucose meters, and/or continuous glucose monitoring systems for the 28 days before hospitalization
Median blood glucose levels for PwT1Ds using CGM systems and those using blood glucose meters are presented in Table II. CGM systems were used by 55 individuals (51.4%). The median times spent in different glucose ranges are shown in Table II. Among PwT1Ds using blood glucose meters, the median number of daily measurements in the 28 days before hospitalisation was 4.6 (Q1–Q3: 3.1–6.5). Detailed data on the number of different blood glucose meter readings are shown in Table II. The use of dual-wave and square boluses was reported by 66 PwT1Ds (61.7%) and confirmed by pump reports for 36 individuals (33.6%). The use of temporary basal rate was reported by 55 PwT1Ds (51.4%) and confirmed by pump reports for 38 PwT1Ds (35.8%).
Laboratory tests
The results of laboratory tests, including median HbA1c and lipid profile, are presented in Table III. An HbA1c target of ≤ 7% was achieved in 31 individuals (29.0%), and an HbA1c target of ≤ 6.5% was achieved in 15 individuals (14.0%).
Table III
Laboratory parameters of metabolic control
Diabetes Treatment Satisfaction Questionnaire
The median DTSQs score was 27 (Q1–Q3: 22.0–29.0), with a score of 4.0 (Q1–Q3: 3.0–5.0) for hyperglycaemia awareness and 2.0 (Q1–Q3: 1.0–3.0) for hypoglycaemia awareness.
Correlations between the evaluated parameters
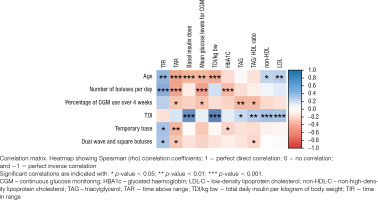
Older individuals had a higher TIR (rs = 0.42, p = 0.001) and a lower TAR > 250 mg/dl (rs = –0.44, p < 0.001), as well as a lower basal insulin dose (rs = –0.37, p < 0.001), TDI/kg bw (rs = –0.4, p < 0.001), and median glucose levels for CGM (rs = –0.36, p = 0.006) than younger individuals. The HbA1c was significantly lower in individuals with a higher median number of boluses per day (rs = –0.33, p < 0.001) and with the use of the temporary basal rate feature (rs = –0.2, p < 0.05). The percentage of CGM use in the 28 days before hospitalisation was negatively correlated with triglyceride levels (rs = –0.35, p = 0.008) and the triglyceride-to-HDL-C ratio (rs = –0.34, p = 0.010). Individuals with higher TDI had higher non-HDL-C (rs = 0.33, p < 0.001), LDL-C (rs = 0.33, p < 0.001), and triglyceride (rs = 0.2, p < 0.05) levels than those with lower TDI. More frequent use of the temporary basal rate was inversely correlated with TAR > 250 mg/dl (rs = –0.37, p = 0.007) and positively correlated with TIR (rs = 0.34, p = 0.012). More frequent use of dual-wave and square boluses was associated with higher TIR (rs = 0.31, p = 0.021) and lower TAR > 250 mg/dl (rs = –0.27, p = 0.045). Correlations are presented in Figure 1.
Discussion
While there have been significant technological advances in the field of diabetes care, these advances alone cannot ensure that the treatment goals for PwT1Ds, as outlined in global guidelines, will be achieved. In recent years, many participants in our study have transitioned from paediatric care to adult care centres. Previous research confirmed that glycaemic control parameters are higher during this transition period. In the PolPedDiab study, Szadkowska et al. [21] demonstrated that more than 40% of Polish children and young adults with T1D achieve the recommended HbA1c target. In our study, only 29% of participants achieved an HbA1c value of ≤ 7%, and the median HbA1c value was higher than that reported by other studies on T1D treatment [22, 23].
On the other hand, we observed improvement in TIR as well as TAR > 250 mg/dl with increasing PwT1D age and age at T1D diagnosis, suggesting that older individuals may have a higher level of self-discipline in managing T1D [24]. However, our study did not find a correlation between the duration of T1D and lower HbA1c values. This is surprising, as one would expect greater experience to improve disease management. Thus, the PwT1D age and age at diagnosis seem to have a significant but insufficient impact on glycaemic control, suggesting that diabetes education should be (tailored to different age groups). Despite advances in treatment, T1D is still associated with an increased risk of mortality [25]. Moreover, in our study, the disease is associated with a significant incidence of acute and chronic complications (11.2% and 12.1%, respectively). Therefore, there is a great need for educational programs in this population. Among PwT1Ds included in our study who experienced hypoglycaemia requiring third-party assistance, only 1 in 4 were using CGM. This indicates that CGM use may be associated with lower risk of severe hypoglycaemic events. Individuals using CGM also had lower triglyceride levels and triglyceride-to-HDL-C ratio, suggesting a lower risk of cardiovascular events and chronic diabetes complications [26, 27]. Median LDL-C levels in the study group met the recommended therapeutic target for individuals with moderate cardiovascular risk; however, these results should be considered individually for each person according to the individual total cardiovascular risk.
In our study, older age correlated with lower TDI and basal insulin dose – parameters that are also associated with a beneficial effect on cardiovascular risk [28]. The daily insulin dose in the study group was consistent with general recommendations, contradicting the notion that pump users tend to increase their insulin dose. The positive correlations between TDI and triglyceride and LDL-C levels confirm the benefits of good glycaemic control and lipid metabolism.
Among less favourable outcomes, most individuals in our study reported lower physical activity level than recommended [29]. Smoking was reported by 19.6% of participants, which is only slightly lower than the global prevalence of smoking [30].
Our study showed that PwT1Ds who use the temporary basal rate and dual-wave and square bolus pump features achieve higher TIR and lower TAR > 250 mg/dl. More frequent use of the temporary basal rate was inversely correlated with HbA1c values. Therefore, the diabetes team should educate PwT1Ds about the need for frequent and appropriate use of this option. It is important to note that participants reported more frequent use of advanced insulin pump features than indicated by the insulin pump reports. The actual use of dual-wave and square boluses and temporary basal rate by 36% and 38% of participants, respectively, suggests that the potential of CSII therapy is not being fully utilised. This may be due to insufficient training, resulting in a lack of confidence in using these features. It was also observed that more frequent bolus administration was associated with lower HbA1c values. Therefore, participants in the GoPump program are trained to use temporary basal, simple, dual-wave, and square boluses.
In the context of diabetes care, motivation for treatment should also be considered, because T1D is associated with a higher prevalence of depression [31]. In our study, we also assessed satisfaction with current diabetes treatment. Participants demonstrated awareness of hyperglycaemia and hypoglycaemia, as shown by the results of the DTSQs. Thus, inadequate metabolic control in these individuals may not be due to unawareness of glucose levels, but rather to an inability to effectively respond to hyperglycaemia and hypoglycaemia.
Proper use of an insulin pump remains a challenge for many PwT1Ds and healthcare providers. This is due not only to the lack of readily available structured diabetes education programs, but also due to the rapid advancement of insulin pump technology. Most people treated with CSII still use traditional pumps rather than the most advanced automated insulin delivery systems. Diabetes education programs were shown to improve treatment outcomes regardless of the treatment modality and the use of new technologies [32–35].
One of the main limitations of this study is its cross-sectional design, which inherently limits the ability to establish causal relationships between the analysed parameters. Additionally, the study cohort was relatively heterogeneous, as not all participants used CGM systems. This may lead to inconsistencies in data quality and comparability, potentially affecting the generalisability of the findings.
This study presented the treatment outcomes of T1D and detailed data on insulin pump programming and the use of various pump features. More research is needed to assess factors affecting glycaemic control in PwT1Ds and to develop more personalised strategies for diabetes treatment and PwT1D education. The effectiveness of the GoPump program will be assessed in future studies with follow-ups at an outpatient diabetes clinic after 6 and 12 months.
Conclusions
In the study cohort, older age was associated with improved outcomes in PwT1Ds. The use of advanced CSII features and administration of more boluses per day appeared to improve diabetes control. However, these advanced features remain underutilised by many PwT1Ds. Our findings suggest that most young PwT1Ds treated with CSII do not meet the criteria for optimal T1D control, highlighting the need for systematic diabetes education.

 POLSKI
POLSKI