A 74-year-old man with arterial hypertension, hypercholesterolemia and a history of surgical treatment of pulmonary artery aneurysm presented with acute chest pain lasting over 15 h. His electrocardiogram showed T-wave inversion in leads V2-V4. Despite hemodynamic stability, he reported persistent chest pain. Transthoracic echocardiography revealed reduced left ventricular ejection fraction of 30% with akinesis of the apex and adjacent segments. Emergent coronary angiography via radial access revealed significant stenosis with extensive thrombus in the proximal left anterior descending coronary artery (LAD) with TIMI grade 2 flow (Figures 1 A, B). Additionally, the circumflex artery showed stenosis up to 60%. The patient was pretreated with unfractionated heparin, aspirin and prasugrel. A hybrid coating guidewire was advanced to the distal LAD using a 6F EBU guiding catheter. Thrombus aspiration was performed using a 6F Export catheter with four passes, successfully retrieving large thrombus fragments (Figure 1 C). For patient stabilization, an intracoronary bolus of abciximab (a glycoprotein IIb/IIIa inhibitor) was administered, followed by deployment of a 3.5 × 38 mm drug-eluting stent at 24 atm, achieving suboptimal sizing (Figure 1 D). Post-deployment imaging revealed slow-flow phenomenon. Intravascular ultrasound (IVUS) excluded dissection but confirmed stent malapposition (Figure I E). Given the impaired coronary flow and prolonged chest pain, stent post-dilatation was deferred. The patient received dual antiplatelet therapy and continuous abciximab infusion for 12 h, followed by therapeutic doses of low molecular weight heparin. Four days later, IVUS-guided percutaneous coronary intervention was performed for stent optimization. High-pressure inflations using non-compliant balloons (4.0 × 20 mm and 5.0 × 15 mm) at 16 atm achieved optimal stent sizing (Figure 1 F). No detriment in the flow was observed. The remaining hospitalization was uneventful. Guideline-recommended heart failure treatment was implemented. The patient was discharged in good condition after 7 days.
Figure 1
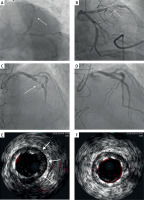
A – Initial coronary angiogram showing significant stenosis with high thrombus burden in the proximal left anterior descending artery (LAD) (arrow) and up to 60% stenosis in the circumflex artery. B – Initial coronary angiogram showing significant stenosis with high thrombus burden in the proximal LAD (arrow) and up to 60% stenosis in the circumflex artery. C – Thrombus aspiration using a 6F Export aspiration catheter (arrow). D – Coronary angiogram after drug-eluting stent implantation into the proximal LAD. E – Intravascular ultrasound (IVUS) imaging demonstrating stent underexpansion (arrows). F – Final IVUS imaging confirming optimal stent apposition after high-pressure post-dilatation
Primary percutaneous coronary intervention in myocardial infarction with high thrombus burden carries a risk of angiographic complications including slow-flow/no-reflow phenomenon and/or distal embolization [1]. Despite angiographically successful aspiration thrombectomy, residual thrombus remains, increasing the risk of distal embolization through thrombus migration to the microcirculation during stent implantation. Furthermore, the presence of a high thrombus burden may affect proper vessel sizing, resulting in stent malapposition and subsequently an increased risk of stent thrombosis [1]. In such clinical scenarios, a deferred stenting strategy was suggested [1, 2]. This approach allows for gradual thrombus resolution, improved microvascular flow, reduced vasospasm, and prevention of distal embolization [1, 3]. However, the clinical benefit of this strategy was not confirmed in randomized clinical studies, and routine use of the deferred stenting strategy is not recommended by the current guidelines [4]. Alternatively, in selected cases, initial undersized stenting followed by delayed IVUS-guided optimization can minimize the risk of complications while achieving optimal stent apposition. Although this strategy extends hospital stays and increases treatment costs, it may represent a safe and effective approach in selected primary PCIs, particularly in cases with high thrombus burden [2].








