Introduction
Interventional endoscopic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP), often require sedation. The most commonly used agent for this purpose is midazolam. This drug has a short elimination half-life and has amnestic and anxiolytic effects [1]. Propofol is a widely used sedative and hypnotic agent with minimal analgesic potential. Propofol may cause respiratory depression, especially in high doses [2]. To reduce cumulative propofol doses, it may be used in combination with other drugs, such as midazolam or dexmedetomidine. Propofol and midazolam act synergistically in combination and may be more effective than when used alone [3]. Dexmedetomidine is a highly selective α2-adrenergic receptor agonist that has analgesic and sedative effects with minimal depression on ventilation [4]. It has been reported that dexmedetomidine reduces the recruitment of propofol during anaesthesia because dexmedetomidine has analgesic effects [5].
The level of sedation can easily shift from conscious to deep sedation and result in the loss of protective reflexes and may cause problems in airway control [3]. Therefore, the sedation level should be monitored and managed carefully. For assessment of the level of sedation, bispectral index (BIS) monitoring may be used; it is an objective method and provides titration of drugs [6, 7]. The BIS is a complex mathematical evaluation of relevant, descriptive electroencephalographic parameters of the frontal cortex corresponding to varying levels of sedation [8]. Patients undergoing general anaesthesia require a BIS level of 40 to 60, and a level of approximately 80 is adequate for less invasive procedures, such as ERCP and endoscopic interventions [9].
Aim
In this study, the primary aim was to compare the recovery time after propofol consumption between the dexmedetomidine-propofol group and midazolam-propofol group in patients undergoing ERCP. The secondary outcome was to compare cardiorespiratory responses of the groups. We hypothesized that a combination of dexmedetomidine-propofol would provide a shorter recovery time than a midazolam-propofol combination without respiratory side effect and haemodynamic instability.
Material and methods
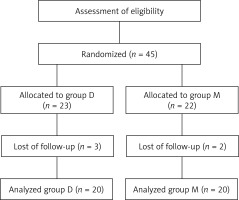
This randomized, prospective and double-blind study was conducted with the approval of the university ethics committee. This study is registered and approved by the University Medical Ethics Committee with the registration number of 04-2009/112, 09.04.2009. We obtained written informed consent from all patients. Forty patients scheduled for therapeutic ERCP aged between 20 and 78 years were enrolled in the study. All patients were in ASA I–III physical status. Patients under the age of 18, those who were pregnant, chronically using opioid or α2 agonist drugs, had a history of allergy to one of the drugs used in the study, had severe cardiac or respiratory comorbidity, had second- or third-degree heart block, had ASA IV-V status, had a body mass index (BMI) over 36 kg/m2 and those who refused to participate in the study were excluded from the study. The patients were randomized to two groups using a computer by a physician who did not follow up the patients during the procedure (Figure 1). After being taken to the operating room, patients were monitored for heart rate (HR), mean arterial blood pressure (MAP), respiratory rate (RR) and arterial oxygen saturation (SpO2), and the baseline measurements of haemodynamic variables were recorded. BIS monitoring (Aspect Medical Systems, Natick, Mass, US) was also applied to all patients. BIS values range between 0 and 100 (0: no cortical activity or coma; 40–60: unconscious; 70–90: varying levels of conscious sedation; 100: fully awake). In this study, the BİS value was maintained at 70–80, which was sufficient for conscious sedation. After recording the basal values, we used a topical anaesthetic (Vemcaine pump spray 10%, VEM Pharmaceuticals Industry and Trade Co., Tekirdag, Turkey) for pharyngeal anaesthesia. Patients were premedicated by a physician who was informed about the randomization. In one group (group M; n = 20), patients were premedicated with midazolam 0.05 mg/kg (IV) 10 min before propofol administration. In the other group (group D; n = 20), patients received dexmedetomidine (Precedex 200 μg, Hospira, Rocky Mount, USA) 1 μg/kg (IV injected in 10 min) prior to the propofol administration. The physician who applied the premedication did not participate in the patient’s follow-up to maintain study blindness. Two other physicians followed the patients after premedication. Propofol (propofol 1% Fresenius Kabi USA, LLC, Lake Zurich) in the same doses (1–1.5 mg/kg for first bolus dose and intermittent doses of 20 mg to achieve a BIS score between 70 and 80) was used for sedation in both groups. ERCP was initiated after achieving an adequate sedation level (BIS: 70–80). The ERCP was performed by an experienced gastroenterologist (over 3000 therapeutic ERCP procedures) in a standard manner. The data (HR, MAP, RR and SpO2) were recorded at 5-min intervals during the procedure. When there was more than a 20% increase or decrease in heart rate and blood pressure, it was evaluated as a side effect. Other side effects (such as arrhythmias, nausea-vomiting and shivering) were also recorded. In the case of bradycardia (HR < 40 beat/min), hypotension (MAP < 50 mm Hg), bradypnea (RR < 10/min) or desaturation (SpO2 < 92%), adequate therapeutic applications were carried out in each situation (atropine 0.5 mg for bradycardia, 0.9% saline infusion (500 ml/h) for hypotension, and oxygen supply (4 l/min) with a nasal cannula for desaturation were the planned treatments). In the event of respiratory depression, patients were planned to be supported with a jaw thrust maneuver and ventilation with a balloon mask. During the procedure, propofol doses were also administered to maintain BIS levels between 70 and 80, and the cumulative dose was recorded. With termination of ERCP, drugs were ceased, and patients were evaluated with an Aldrete score [10] for defining the recovery period by physicians blinded to the drug regimen. The period between the termination of ERCP and time to achieve an Aldrete score of 9 was accepted as the recovery time.
Statistical analysis
The data were analysed using the IBM SPSS Statistics for Windows, version 22 (IBM Corp., Armonk, N.Y., USA) program. All data were analysed in terms of mean ± standard deviation (SD). After the preliminary study with 10 patients, we performed a power analysis by using the recovery time. We calculated the sample size as 26 in total (α = 0.05; power = 0.95). We performed this study with 40 patients, and post hoc power analysis (G Power 3.1.9.2) according to recovery time with 40 patients was performed; for α = 0.05 and power = 0.99, the effect size was 1.897. For continuous variables, Student’s t test was used to compare differences between groups. Category variables were analysed with the χ2 or Fisher’s exact test as appropriate. For all tests, a p-value < 0.05 was considered to be significant.
Results
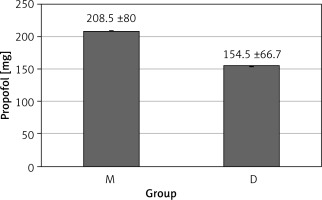
Forty patients were enrolled in the study. The demographic parameters of patients were similar between groups. The age, gender, height and body weight, ASA physical status and operation time are shown in Table I. Changes in blood pressure and heart rate were similar in both groups (Figures 2 and 3). When the groups were compared according to the recovery time with the Aldrete score, the recovery time to achieve the Aldrete score 9 was found to be shorter in group D than in group M (6.6 ±1.1 min in group D vs. 9.4 ±2.1 min in group M, respectively; p < 0.001). Additionally, eye-opening, verbal response and cooperation times were significantly lower in the dexmedetomidine group, as shown in Table II. In both groups, BIS values of 70–80, which indicate the targeted sedation level, were reached. The mean ± SD BIS scores are shown in Table III. Total propofol consumption was compared between groups. In the midazolam group, cumulative propofol doses were significantly higher (208.5 ±80.0 mg vs. 154.5 ±66.7 mg; p < 0.01) than in the dexmedetomidine group (Figure 4).
Table I
Demographic variables of patients in groups
Table II
Recovery time variables between groups
| Parameter | Group M | Group D | P-value |
|---|---|---|---|
| Time to reach Aldrete score 9 [min] | 9.4 ±2.1 | 6.6 ±1.3* | < 0.001 |
| Eye opening time [min] | 6.9 ±1.9 | 4.8 ±1.5* | < 0.001 |
| Verbal response [min] | 8.5 ±2.0 | 6.1 ±1.5* | < 0.001 |
| Cooperation time [min] | 10.3 ±2.7 | 7.7 ±2.0* | < 0.001 |
Table III
Mean BIS values in groups
Figure 2
Changes in heart rate (HR) values in two groups
Values are expressed as mean ± SD. Baseline (before sedation), Psprem (after premedication), Pro 1. min (1st min of the procedure), 5. min (5th min of the procedure), 10. min (10th min of the procedure), 15. min (15th min of the procedure), 20. min (20th min of the procedure).
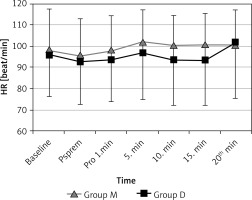
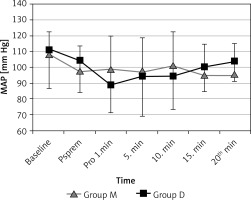
Figure 3
Changes in mean arterial blood pressure (MAP) values in two groups
Values are expressed as mean ± SD. Baseline (before sedation), Psprem (after premedication), Pro 1. min (1st min of the procedure), 5. min (5th min of the procedure), 10. min (10th min of the procedure), 15. min (15th min of the procedure), 20. min (20th min of the procedure).
When hemodynamic variables were analysed with in-group analysis, we observed that heart rate values decreased after premedication and after propofol bolus injection in both groups. This decrease was an expected effect secondary to the sedation. However, the decrease did not exceed a level that was 20% over baseline values, as shown in Figure 2.
There was no arrhythmia, apnoea, shivering or bradycardia (> 20% decrease to baseline) in either group. Hypotension occurred in 1 patient in group M and 3 patients in group D (p = 0.302). Nausea/vomiting was observed in 3 patients in group M and none in group D (p = 0.231). Five patients in group M and 4 patients in group D needed oxygen supply (25% vs. 20%, respectively; p > 0.05). Side effects were similar, as shown in Table IV.
Table IV
Adverse effects in groups
Discussion
In this study, we found that patients receiving dexmedetomidine-propofol sedation during ERCP had a significantly shorter recovery time. Less propofol consumption in the dexmedetomidine group was thought to have affected this result. The changes in respiratory and haemodynamic variables were similar in both groups.
Adequate sedation during endoscopic procedures, especially for interventional ERCP, directly affects the operation time and success. Propofol is widely used for this purpose, and it is postulated to be effective in sedation for ERCP. In a meta-analysis, propofol-induced respiratory depression and hypotensive effects were shown to be more common than in single use. In the same article, it was reported that recovery time was shorter and patient cooperation was better when used with opioids or midazolam [11]. However, higher doses of propofol may cause platelet aggregation [12], metabolic acidosis [13], delayed awakening [14], depression of the hypoxic ventilator response, and cardiorespiratory depression. Therefore, to decrease these adverse effects of propofol, it is commonly combined with other sedatives. Peden et al. [15] reported that the addition of dexmedetomidine to propofol caused a reduction in the propofol requirement and a decrease in the plasma concentrations of propofol. In our study, we found statistically significantly lower propofol consumption in the dexmedetomidine group.
In a study, it was demonstrated that conscious sedation for diagnostic and therapeutic ERCP can be successfully and safely achieved using midazolam [16].
In a randomized, double-blind, prospective clinical trial, the authors compared propofol plus fentanyl with dexmedetomidine infusion alone. These researchers found that patients needed more additional analgesics in the dexmedetomidine group. The authors also concluded that dexmedetomidine was associated with higher haemodynamic instability and prolonged recovery [17]. This finding is different from the findings of our study. We think that this difference may be due to high doses of dexmedetomidine, which was used alone during the entire procedure in that study. In our study, we only applied dexmedetomidine or midazolam before the procedure. Sedation maintenance was supplied with propofol. The recovery time was shorter in our study because we used dexmedetomidine in combination with a lower cumulative dose of propofol. Both the lower doses of dexmedetomidine and propofol may be effective for shorter recovery times. Seifert et al. [3] compared propofol alone and propofol-midazolam combinations in interventional endoscopy. The authors found similar sedative efficacy in both groups but longer recovery times in the propofol-midazolam combination group (19 ±7 vs. 25 ±9 min; p < 0.01). This finding may be due to the relatively slower elimination half-life of midazolam. In our study, the shorter recovery time in the dexmedetomidine group may be secondary to both the short elimination half-life of dexmedetomidine and less propofol consumption.
A study by Lee et al. [18] compared the sedative effect and adverse events of midazolam–meperidine–dexmedetomidine and midazolam–meperidine during ERCP and found that adding dexmedetomidine to the midazolam–meperidine regimen was more effective and safe during ERCP compared with a midazolam–meperidine regimen.
We used BIS measurements to objectively achieve adequate sedation levels. Thus, the total sedative agent dose did not depend on the operator’s subjective evaluation. We found that the total propofol consumption was lower in group D (154.5 ±66.7 vs. 208.5 ±80.0 mg; p = 0.011). This result is important, and it may be postulated that dexmedetomidine might be a good alternative for sedation with the propofol sparing effect. It can be postulated that due to less propofol consumption in the dexmedetomidine group, cost-effectiveness in this group is better. This approach reduces the possible respiratory depressive effect of propofol by decreasing the total consumption. In an experimental study with a rabbit model, the researchers found that ventilator depression was higher in treatment with propofol and midazolam. The depression was lowered with dexmedetomidine [19]. This outcome is an important respiratory protective effect for ambulatory sedation.
Dexmedetomidine has been associated with decreases in HR because of its α2 agonism and sympatholytic effect [20]. In a study, significant decreases in MAP and HR occurred if dexmedetomidine was used as monotherapy [17, 20]. This effect may be due to the higher doses of the drug, and decreased sympathetic outflow and circulating catecholamine levels [21]. Gastrointestinal endoscopy studies using dexmedetomidine and midazolam showed that the two agents do not differ from each other in terms of hypoxia, bradycardia and hypotensive effects [22]. Again, in this meta-analysis, patients who were treated with dexmedetomidine for longer procedures, such as ERCP or endoscopic mucosal resection, reported less restlessness [23]. In our study, we did not find any respiratory or hemodynamic differences (MAP and HR) between the groups. In group analysis, the MAP decreased during the procedure but did not exceed 20% compared to the baseline values. The most important cause of hemodynamic side effects due to dexmedetomidine is the high-speed and long-term induction dose [24]. It has also been reported that it may have hypertensive effects when used as the sole agent [25]. In our study, dexmedetomidine was used in induction together with propofol at one time and 1 μg/kg (10 min). Infusion treatment was not given. A hemodynamic side effect was not clearly observed due to the reasons mentioned above.
Our study has limitations. Recovery times between the two groups are very similar. The reason for this is that we have worked with a very experienced (> 3000 cases) gastroenterologist because the duration of the procedure in our study is relatively short compared to other studies [22].
In a study in which propofol was used in combination with dexmedetomidine and midazolam, side effects such as restlessness and agitation were observed as a side effects Purshka. These effects are insufficient sedation findings. In this study, sedation may not be evaluated objectively because sedation was performed according to the Ramsey sedation score. When the side effects were examined in our study, no difference was observed in terms of restlessness between the groups, since sedation was achieved at the same level as BIS monitorization.
There was no difference in nausea-vomiting between the groups because antiemetic treatment was applied in both groups.
Conclusions
In this randomized, double-blind, prospective study, we found that the dexmedetomidine-propofol combination had a shorter recovery time and similar sedative and adverse effects compared with the midazolam-propofol combination. On the other hand, our study suggests that adjunctive use of dexmedetomidine reduces propofol requirements during ERCP. Therefore, we can conclude that the combination of dexmedetomidine and propofol may be a safe and useful alternative for sedation and dose reduction in ERCP patients.