Introduction
Diabetes is a well-known risk factor for postoperative complications, which can prolong hospital stays, consume healthcare resources, and increase mortality. One of the most serious complications is an increased risk of infection during the perioperative period. Data show that hyperglycemic states with blood glucose levels of ≥ 200 mg/dl reduce leukocyte function and inhibit protection against infectious diseases [1, 2]. Furthermore, in severely ill patients, an elevated metabolism has been suggested to increase insulin resistance and cause stress hyperglycemia [3, 4]. However, although strict glycemic control with intravenous glucose-insulin therapy has been reported to be beneficial [3, 4], whether hepatectomy with strict glycemic control is safe and mandatory for diabetic patients remains controversial.
Herein, we report the preoperative conditions, perioperative outcomes, and postoperative courses of hepatectomies performed on patients with diabetes, a comorbidity that has been increasing in recent years, along with a short discussion of the literature.
Material and methods
Patient population and selection
We retrospectively reviewed the data of 363 consecutive patients who underwent laparoscopic and open hepatic resection for hepatocellular carcinoma (HCC) at Osaka Medical College Hospital, Takatsuki City, Japan between January 6, 2010 and December 25, 2018. A total of 358 patients who underwent liver volumetry at three time points were finally included in this study. All patients were fully informed of the study design and provided written informed consent for participation. The study design was approved by the Ethics Committee on Clinical Investigation of the Osaka Medical College Hospital (approval numbers 2001 and 2059).
Surgical procedure
The laparoscopic and open surgical techniques routinely used in our department have been described previously [5–8]. Parenchymal transection was performed using a surgical tissue management system (Thunderbeat, Olympus Inc., Tokyo, Japan) and a Sonop 5000 ultrasonic dissector (Hitachi Aloka Medical, Ltd., Tokyo, Japan). Small vessels were ligated or coagulated using a soft-coagulation system. Intraparenchymal control of major vessels was achieved with nonabsorbable sutures, while biliary and vascular radicle division was accomplished with stapling devices or nonabsorbable sutures. The hepatic pedicle was always isolated to enable the Pringle maneuver by inhibiting the blood flow with a vascular occlusion tube (Vessel-Clude; Argon Medical Devices Inc., Frisco, TX, USA), if possible.
Evaluation of liver volume measurements and visceral fat area
The volume analyzer Synapse Vincent image analysis system (Fujifilm Medical, Tokyo, Japan) automatically calculated the approximate total liver volume (TLV) on the preoperative computed tomography (CT) scans. Remnant liver volume (RLV) was measured using the MDCT at 7 days, and 1, 2, 5, and 12 months postoperatively. RLV immediately after surgery was calculated as (TLV + tumor volume) – resected liver volume, while the regeneration rate was calculated as (RLV at 7 days, and 1, 2, 5, and 12 months/TLV) × 100. The approximate visceral fat area (VFA) at the umbilical level on preoperative CT scans was also automatically calculated using the Synapse Vincent image analysis system.
Definitions
For the purposes of this study, diabetes mellitus was defined as a fasting plasma glucose level of > 7.0 mmol/l (126 mg/dl), a plasma glucose level of > 11.1 mmol/l (200 mg/dl) measured in a 75-g oral glucose tolerance test, or the need for insulin or an oral hypoglycemic drug to control glucose levels. In addition to the previously listed plasma glucose values, hemoglobin A1c (HbA1c) has been given a more prominent position as one of the diagnostic criteria, i.e., HbA1c levels of ≥ 6.5% is also considered to indicate diabetes [9].
In our study, the target value for pre- and postoperative glycemic control was set to ≤ 200 mg/dl. After hospital admission, blood glucose was measured 4 times a day, with glucose levels controlled using insulin injections each time.
Statistical analysis
To minimize the influence of potential confounders on selection bias, propensity scores were generated using binary logistic regression. The variables were age, sex, body mass index (BMI), pathological diagnosis, viral hepatitis infection status, presence of diabetes mellitus, total bilirubin levels, albumin levels, prothrombin time (PT), platelet count, indocyanine green retention rate at 15 minutes (ICG-R15), Child-Pugh classification, tumor number, largest tumor size, tumor location, and number of hepatic resections. One-to-one matching between groups was accomplished using the nearest-neighbor matching method performed without replacement, using a caliper width of 0.2 standard deviations of the logit of the estimated propensity score. All statistical analyses were performed using JMP version 14 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient demographics
Hepatectomy was performed on 363 patients who were classified into 2 groups: 136 with diabetes (37.5%) and 227 without diabetes (62.5%) (Table 1). Although surgery was not postponed due to severe diabetes, 15 patients (4.1%) with HbA1c levels of ≥ 8.5% were hospitalized a week before the operation for glycemic control. To reduce bias from patient baseline characteristics, we used propensity score matching (PSM).
Table 1
The baseline characteristics and surgical outcomes of the study population underwent hepatic resection
[i] Data was presented as median (range). * p < 0.05. a percentage (%) of the group, PSM – propensity score matching, DM – diabetes mellitus, HCC/CCC – hepatocellular carcinoma/cholangiocellular carcinoma, BMI – body mass index, HbA1c – hemoglobin A1c, PNI – prognostic nutritional index, VFA – visceral fat area, ICGR-15 – indocyanine green retention rate at 15 min, CD – Clavien-Dindo, PHLF – post-hepatectomy liver failure.
In terms of baseline characteristics, the diabetes group consisted of patients who were older (p = 0.002), had a higher BMI (p = 0.004), and had a lower prevalence of viral hepatitis (p < 0.001). HbA1c and fasting plasma glucose levels were significant higher in the diabetic group (p < 0.001 and 0.025). Based on PSM, diseases underlying a brain disease were significantly more common in the diabetic group (11.1%, p = 0.016). One hundred and eight cases were selected for each group with no significant differences observed in surgical outcomes including surgery duration, bleeding amount, and the number of patients with postoperative complications (Clavien-Dindo grade > IIIA) between the groups (p = 0.948, 0.286, and 0.736, respectively). More specifically, there were no significant differences between the groups in the rate of infectious complications, such as superficial incisional, deep incisional, and organ/space surgical site infection, perihepatic abscess, and remote site infection (p = 0.651, 0.995, 0.824, 0.313, and 0.313, respectively). No cardiovascular complications were noted. There were no differences in the incidence of postoperative bile leakage, post-hepatectomy liver failure (PHLF), or intractable ascites (p = 0.422, 0.353, and 0.810, respectively), and the difference in postoperative hospital stays was not significant (p = 0.450).
In the postoperative blood sampling, total bilirubin, serum albumin, PT, platelet counts, aspartate transaminase, and alanine transaminase were not significantly different between the groups, especially on the peak day (p = 0.358, 0.447, 0.902, 0.856, 0.657, and 0.622, respectively).
There was no difference in the remnant liver volume regeneration in either group until postoperative day 7 and month 1, 2, 5, and 12 (p-values: 0.076, 0.368, 0.864, 0.288, and 0.063, respectively, Table 2). With respect to prognosis, there was no significant difference in the overall or recurrence-free survival rates between the two groups (p-values: 0.613 and 0.937).
Table 2
Resected liver volume and remnant liver regeneration
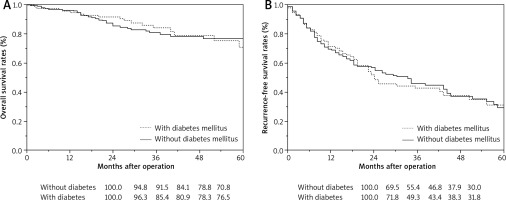
Fig. 1
Surgical outcomes (A) OS, (B) RFS. The 1-, 2-, 3-, and 5-year OS rates with diabetes mellitus were 96.3%, 85.4%, 80.9%, and 76.5%, respectively. The 1-, 2-, 3-, and 5-year OS rates without diabetes mellitus were 94.8%, 91.5%, 84.1%, and 70.8%, respectively. The 1-, 2-, 3-, and 5-year RFS rates with diabetes mellitus were 71.8%, 49.3%, 43.4%, and 31.8%, respectively. The 1-, 2-, 3-, and 5-year RFS rates without diabetes mellitus were 69.5%, 55.4%, 46.8%, and 30.0%, respectively. There were no significant differences in OS and RFS between the with and without diabetes mellitus groups after hepatic resection (p = 0.613 and 0.937)
Discussion
A study on the death causes among 18,385 diabetes patients revealed that the top causes included malignant neoplasms (34.1%), vascular disease (26.8%), and infections (14.3%) [10, 11]. Among malignant neoplasms, HCC is one of the top causes of death, accounting for 8.6% of all deaths among these patients. In the mechanism of carcinogenesis, the activation of insulin-like growth factor (IGF) signals that accompany insulin resistance and hyperinsulinemia is considered important [12]. IGF binds with and activates insulin receptors and IGF receptors. These receptors then activate cell proliferation signals and antiapoptotic signals in hepatocytes to promote carcinogenesis. In addition, hyperglycemic states lead to increased production of oxidative stress, e.g. via an overload of glucose oxidase in mitochondria. In turn, oxidative stress, known to cause vascular damage in diabetes, induces gene mutations from oxidative DNA damage to induce carcinogenesis [13].
The proper management of blood glucose levels has been deemed crucial for controlling infections in the perioperative periods. This involves controlling hyperglycemic states which reduce neutrophil function, phagocytic ability of granulocytes, intracellular bactericidal activity, and immune function and cause coagulation and fibrinolytic system abnormalities. Moreover, persistent hyperglycemia has been shown to induce infections. In particular, HCC patients undergoing hepatectomy and other liver surgeries often experience chronic glucose metabolism disorders, such as cirrhosis, and are in a state of hepatogenous diabetes, which makes perioperative glycemic control difficult [14]. In addition, many patients experience complicated ischemic heart disease, which sharply increases the risks associated with surgery.
In regular elective surgeries, we find that frequent blood glucose monitoring and continuous intravenous insulin administration in the general ward is extremely difficult and rather dangerous. However, we believe that such drastic measures are unnecessary. Therefore, for perioperative glycemic control in our study, we considered measuring blood glucose 4 times a day until the hemodynamics stabilized and controlling glucose levels with insulin injections sufficient. Thus, for perioperative glycemic control close to hepatectomy, we used a target blood glucose level of ≤ 200 mg/dl. As a result, we were not only able to control surgical infections, but also to keep the rate of perioperative complications low, with similar surgical outcomes as for non-diabetes patients [15].
Previous studies on hepatectomy in patients with damaged livers, such as those with impaired glucose tolerance, have reported an impaired regeneration of the remnant liver [16]. However, in our study, remnant liver regeneration did not differ between the groups and the remnant liver was regenerated to the preoperative volume, which highlights important results. From the functional perspective, blood test results returned to the normal range with no differences when compared to baseline. Although diabetes patients may be anxious about undergoing hepatectomy due to a potential risk for postoperative complications, our study indicates that there is no difference in the postoperative remnant liver regeneration. Furthermore, out study highlights that providing that an adequate remnant liver volume is secured and appropriate perioperative management supplied, we believe hepatectomy can be performed on diabetic patients with adequate safety. Additionally, considering the long-term prognosis of diabetes patients, there was no difference between the groups in terms of the relapse-free or cumulative survival rate. Hence, diabetic patients can be expected to have similar prognoses as non-diabetes patients.
The purpose of a preoperative diabetes assessment is not merely to obtain information necessary for a safe surgery, but to also formulate a comprehensive treatment plan for patients who are diabetic. In addition to the severity of diabetes, many additional factors deserve comprehensive consideration, including the patient’s age, degree of invasion of the planned surgery, prognosis, quality of life, and the presence of other serious complications. In the case that a patient is not severely diabetic and a low-risk surgery is planned, a strict management of diabetes may not be necessary. However, in case of severe diabetes, a detailed preoperative assessment is essential, and surgery should only be performed after the patient is stabilized with treatment. Moreover, it may be necessary to work with metabolic and internal medicine specialists to conduct detailed examinations and possibly modify the treatment plan. While performing a hepatectomy according to plan is essential, some patients require prior treatment for diabetes, and modifying the treatment plan may ensure a positive long-term prognosis. In case this has no impact on the surgery, it may be helpful for systemic postoperative management.
Although it is possible to perform hepatectomies safely by modifying surgical techniques, the number of patients with diabetes will increase as society ages, which makes thorough preoperative assessments crucial. While the safety of hepatic resection in diabetic patients has been debatable, there may be no noticeable difference in the postoperative course. However, in cases of severe diabetes, the comorbidity may not be stable peri-operatively, and thus, patients should receive prior treatment for diabetes.








