Introduction
Accidental or suicidal ingestion of a chemical corrosive substance can result in gastrointestinal and respiratory burns. The circumstances of the incident and the chemical nature of the substance determine the extent of injury and toxicological risk. The initial period after a chemical burn is associated with the possibility of laryngeal oedema, perforation of the oesophagus, stomach and intestine, gastrointestinal bleeding and pancreatitis [1–3]. Severe upper gastrointestinal burns affect 10–33% of adult patients, with mortality rates of up to 10% [3, 4]. Patients requiring either an emergency oesophageal resection due to mediastinitis or a gastrointestinal resection due to peritonitis have a more severe course of illness, with a higher risk of death. In this group, perioperative mortality ranges from 15% to 60% [5, 6]. Late sequelae of irritant burns include retrograde changes in the oral cavity, oesophagus, stomach or respiratory system. Furthermore, the healing process may lead to stricture formation within these organs. Stricture formation may lead to severe systemic sequelae for the patient, including poor general condition, significant weight loss, malnutrition-related illness, recurrent aspiration leading to respiratory infections, and potentially respiratory failure. Patients usually require preparation for elective surgery, including reversal of any metabolic disturbances and optimisation of respiratory function. The most common late complication of endoscopically confirmed chemical oesophageal burns is stricture formation, which occurs in approximately a quarter of hospitalised patients [7]. Oesophageal strictures resulting from chemical burns are considered more difficult to treat both endoscopically and surgically than post-reflux or neoplastic strictures [8].
Epidemiology
Chemical burns of the oesophagus are estimated to affect several tens of thousands of people per year, of which 80% are in children under five years of age [1–5]. According to the Annual Report of the American Association of Poison Control Centers’ National Poison Data System, 5–15 thousand oesophageal burns are reported annually in the USA, with an incidence of 1.08/100,000 [9]. Accidents involving children usually occur in the home and typically involve ingestion of a small amount of a chemical substance. Ingestion of corrosive substances by adults tends to have a suicidal background or is the result of a mistake. The amount ingested by adults tends to be higher than in children, and leads to more significant injuries of the upper gastrointestinal and respiratory tracts. Similarly, ingestion of toxic substances in industrial settings, where substances are found in higher concentrations, has more severe consequences [1, 10]. Table I provides a summary of the chemicals that most commonly lead to burns.
Pathophysiology
Ingestion of acids and bases, despite their different pathomechanisms of injury, lead to similar consequences; alkalis more commonly damage the respiratory tract and proximal part of the oesophagus, whereas acids tend to damage the oesophagus, stomach, duodenum and small intestine [1, 3, 10]. The extent of injury to the gastrointestinal and respiratory tract organs is determined by the volume, concentration, molarity and hydrophilicity of the ingested substance, and its duration of contact with tissue. Contact of a base with tissue induces diffuse necrosis, hydrolysis of lipids and damage to the mucosa. The base quickly penetrates deep into the organ wall, causing an intense inflammatory reaction within the immediate and surrounding tissue. Deep damage by acids is exacerbated by intravascular coagulation, as acids cause tissue dehydration and protein denaturation, leading to necrosis and superficial clot formation. Superficial clot formation and the natural properties of the stratified squamous epithelium may provide protection to the deeper layers of the oesophagus [3, 10]. However, this view is challenged by studies that show no significant differences in the depth of gastrointestinal injury in relation to the causative agent [10]. Deep gastrointestinal wall damage results from the direct action of the substance and the subsequent inflammatory process, including thrombosis of submucosal vessels and bacterial colonisation.
Symptoms
Ingestion of a corrosive substance in small amounts may be asymptomatic and may not have any significant sequelae. In 70% of burns of the oral cavity, the oesophagus remains unaffected [1]. Among adults, 10–33% of burns have a severe course requiring treatment, with a mortality rate of up to 10% [3, 4]. Post-burn oesophageal stenosis is found in approximately 1–2% of burn patients [3, 4]. Among patients hospitalised with endoscopically confirmed oesophageal burns, oesophageal stenosis is the most common complication, affecting 24% of patients. Other complications include aspiration pneumonia in 11.36% and respiratory failure in 7.69% [9, 11].
Ingestion of a corrosive substance not only may cause burns but also may have toxicological effects. Acid poisoning may lead to kidney and liver failure, haemolysis and intravascular coagulation. Hydrogen fluoride poisoning may cause severe hypocalcaemia. Phenol, zinc chloride and mercuric chloride are also toxic corrosives. Therefore, the nature of the causative agent and its toxicological profile must also be considered during treatment [1, 3, 10].
It can be difficult to estimate the severity of burns based on clinical symptoms; therefore, patients often require advanced diagnostics [12].
Diagnosis
Laboratory testing
In general, there is no correlation between the results of laboratory tests and the severity of oesophageal burns. Leukocytosis on admission > 20,000/mm3 is one of the parameters which correlates with a high risk of death [13]. pH < 7.22 and BE < –12 are considered markers of severe post-burn oesophageal damage.
Chest X-ray and abdominal X-ray
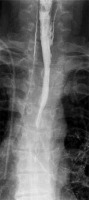
An overview chest X-ray and abdominal X-ray on admission are used as part of the initial assessment. Chest X-ray may reveal oesophageal perforation, pneumomediastinum, pleural fluid or pneumothorax, and abdominal X-ray may show free air under the diaphragm, indicating gastroesophageal perforation. Subsequent chest X-ray examinations are used to assess for pulmonary complications. Oesophageal perforation may also be diagnosed by the presence of extra-oesophageal streaking of aqueous contrast (Figure 1).
Computed tomography (CT)
Neck, chest and abdominal CT are standard examinations in patients with upper gastrointestinal burns to investigate for perforation or partial oesophageal wall damage, which can have severe consequences [1]. Studies by Bruzzi et al. and Ryu et al. demonstrated the high sensitivity of CT in assessing damage to consecutive layers of the oesophagus and suggest that CT evaluation with contrast can replace endoscopic examination, which often cannot or should not be performed in the acute phase of burns. CT can also assist in distinguishing patients who are at risk of developing oesophageal obstruction (Table II) [14, 15].
Table II
Oesophageal burn grading based on CT findings, based on Ryu et al. [14]
Patients with CT findings consistent with grade 3 or 4 oesophageal burns are at greater risk of developing oesophageal stenosis [14] (Table III).
Table III
Oesophageal burn grading based on CT findings, based on Bruzzi et al. [15]
In a study by Bruzzi et al. [15], the risk of developing stenosis in grade I, IIa and IIb burns was 0%, 17% and 83%, respectively. Patients with grade III burns typically underwent oesophageal resection and were thus excluded from the study.
CT findings indicating full-thickness necrosis during the initial phase of the burn have also been established as a predictor of post-burn stenosis. The authors demonstrated that a non-contrast phase CT image in keeping with a grade 4 burn, according to Ryu et al., showing blurring of the boundaries between layers of the oesophageal wall, obliteration of the perioesophageal tissue, and lack of post-contrast enhancement of the oesophageal wall, is a reliable predictor of post-burn stenosis [2, 15].
Endoscopy
Considering the pathophysiology of oesophageal burns, it is deemed safe to perform oesophagogastroduodenoscopy (OGD) up to 48 or even 96 hours after the burn in order to perform an early assessment [1]. In children, deep sedation or general anaesthesia is required, and therefore indications for endoscopy in a child should be considered on an individualised basis. Routine endoscopy in children is supported by the results of studies demonstrating the presence of severe oesophageal injuries in 12–35% of asymptomatic cases [16]. Deep lower pharyngeal burns, severe burns of the respiratory tract, poor general condition and evidence of gastrointestinal perforation on imaging studies are considered relative contraindications for OGD. Endoscopic assessment of the injury makes it possible to predict the severity of the course and the risk of complications, including the development of stenosis [17]. The most commonly used oesophageal burn scale used in endoscopy is described by Zargar’s studies [1, 11, 17] (Table IV).
Table IV
Burn grade based on endoscopy findings, based on Zargar
Studies involving endoscopic examination have demonstrated that serious complications such as bleeding, fistula formation and perforation can be expected in grade IIa–III burns. Oesophageal strictures in both children and adults are formed following burns of at least grade IIa, and most commonly in grades IIIa and IIIb [11].
Treatment
The guidelines currently available are based on the results of a small number of studies, usually retrospective, without randomisation, or a case series [1, 2]. Therefore, they do not hold significant weight in terms of evidence-based medicine. In an attempt to systematise current knowledge, the World Society of Emergency Surgery (WSES) issued a set of recommendations in 2015 [2]. These recommendations suggested that the management of acute oesophageal burns in most cases is conservative.
First, the risk of laryngeal oedema should be assessed and, if dyspnoea is increasing, intubation or tracheostomy should be performed [1]. At the scene of the accident, the type and amount of substance ingested should be determined, as well as the nature of the event (suicidal, accidental). Vomiting should not be induced, nor should antacids be used due to the risk of secondary burn with regurgitated gastric contents or as a result of an exothermic reaction [2].
Early endoscopy (up to 48 hours, but preferably within 6–12 hours) combined with CT scanning allows assessment of the extent of the burn, the need for emergency surgical treatment and the risk of oesophageal stenosis [1–3]. Patients without features of oesophageal burns, or with burns in grades I or IIa on the Zargar scale, and with no significant burns of the oral cavity, pharynx or larynx, can be discharged from hospital after 48 hours of observation [18]. Patients with greater than grade IIa burns require prolonged inpatient observation or hospitalisation in an intensive care unit.
Indications for emergency oesophageal resection include:
oesophageal perforation,
mediastinitis, or
compatible CT and endoscopic findings (grade IIIb burns) indicating full-thickness necrosis. If the CT result is not compatible with endoscopic imaging, conservative treatment is indicated [2]. The need for abdominal intervention is determined primarily by peritoneal symptoms or massive haemorrhage. During emergency laparotomy, the aim should be to remove all damaged organs (e.g. stomach, and if necessary, duodenum, pancreas, gallbladder, small bowel and transverse colon) [2, 5] The efficacy of a two-stage procedure (the so-called “second look” surgery) has not been proven, as there is inevitable penetration of the corrosive substance through the organ walls within the first hours after the burn and therefore all affected tissues should be removed during one procedure [2].
Pharmacotherapy and prevention of stenosis
Proton pump inhibitors are routinely used, but there is no evidence that prophylaxis of acid reflux significantly reduces the risk of oesophageal stenosis. A gastric probe is also routinely used, which helps to decompress the stomach and provides a route for nutrition. The presence of the probe prevents complete scarring of the oesophageal lumen and allows subsequent dilatation procedures. However, it is possible that the presence of the probe may trigger a foreign body reaction and gastroesophageal reflux, and therefore may promote the development of stenosis [1, 2, 18]. Currently, routine use of steroids is not recommended except for indications arising from pulmonary complications [2]. Broad-spectrum antibiotic therapy is often used together with steroids in the treatment of pulmonary complications and as part of the management of septic shock [2, 18].
Zargar grade IIa–IIIb burns are likely to lead to oesophageal or gastric strictures, which require endoscopic treatment as a first step. These strictures are classified as complex due to their length > 2 cm, multilevel nature and tortuous course. The efficacy of dilatation of post-burn stenoses is lower than dilatation performed due to reflux, and the risk of perforation is higher (0.4–32%) [1, 2, 18]. Endoscopic dilatation is commended around three weeks after the burn, with subsequent procedures repeated every 1–3 weeks [1, 2]. Studies on the local administration of steroids (triamcinolone) or antimetabolites (mitomycin C), as well as stenting of the oesophagus, have been shown to prolong the dysphagia-free time between successive dilations, and in a few cases, eliminated the need for further interventions [1, 2, 8, 18]. Post-burn stenosis is estimated to develop a minimum of six months after the burn, after which time surgical treatment can be considered [2, 19].
Surgical treatment of oesophageal stenosis
Planning of surgical treatment of patients with post-burn stenosis of the oesophagus or stomach requires assessment of the patient’s nutritional status, the level and length of the stenosis, any concomitant injuries, including strictures of the oral cavity, pharynx and larynx, and the presence of fistulas involving the bronchial tree [2, 4]. Post-burn stenosis of the pyloric part of the stomach precludes the use of this region during reconstructive surgery. Impaired gastric emptying is an indication for antrectomy or small bowel bypass anastomosis [2]. Post-burn oesophageal stenosis requires oesophageal resection or bypass surgery. Oesophageal resection may be preferred due to the increased risk of tumour development in the burn scar [20–22]. Considering that neoplastic transformation occurs after 20–40 years, oesophageal scar removal is justified mainly in children and young adults [2]. Oesophageal bypass surgery without resection is particularly popular in Far Eastern countries [23]. Bypass is acceptable where the oesophageal obstruction is located at or above the level of the upper thoracic orifice and in cases of incomplete oesophageal obstruction, where the distal segment drains to the stomach. Leaving an obstructed portion of the oesophagus in the thoracic segment may lead to the development of mucocele, which can exert pressure on adjacent organs and lead to abscess formation [24]. Oesophageal resection is performed either via thoracotomy or transperitoneally. Transperitoneal access is reserved for strictures located below the tracheal bifurcation [23, 25]. In bypass surgery, neck anastomosis is typically performed via the retrosternal route in the anterior mediastinum.
Historical overview
Successful oesophageal resections and reconstructions have been reported since the end of the 19th century. In 1900, Jan Mikulicz-Radecki performed the world’s first transperitoneal oesophageal resection in a clinic in Wroclaw. He was also a pioneer of plastic surgery, treating stenosis of the cervical portion of the oesophagus using skin flaps, performing the first such procedure in 1886 [26]. In 1907, Roux described successful oesophageal reconstruction following partial excision using a loop of the small intestine. This method was limited by the inability to create an intestinal segment greater than 30 cm in length, with longer segments resulting in conduit necrosis in about 20%. The mortality rate in the 1930s was as high as 46%. In 1946, Longmire improved the Roux technique by adding microvascular anastomoses of the mesenteric intestinal vessels with the internal thoracic vessels. The development of microsurgery techniques in the second half of the 20th century also contributed to the development of methods using pediculed or free small intestine flaps for oesophageal reconstruction, even in the cervical segment [27]. In 1911, Vuillet and Kelling independently presented the anatomical basis for using the large intestine for the oesophageal reconstruction procedure. In 1914, Von Hacker described the first successful oesophageal reconstructive surgery using the large intestine. In 1951, Orsoni performed the first simultaneous oesophageal resection and reconstruction using the large intestine. In 1965, Belsey published a case series of 104 patients treated with an isoperistaltic loop of the left colon where the overall mortality rate was 4.8%, following which this technique became widely used [28, 29]. The advantage of this particular technique was the ability to perform high anastomoses, including colopharyngeal anastomoses. However, it was not until 1978 that Akiyama demonstrated that the stomach can also be successfully used for anastomoses with the cervical section of the oesophagus and pharynx [28, 30]. Nowadays, it is the stomach which is the organ most frequently used in oesophageal reconstruction operations, including oncological cases [28, 29]. The work of Jezioro and his students from the Department of Gastrointestinal Surgery in Wrocław, which began in the 1960s, has significantly contributed to the development of oesophageal surgery in Poland. In this centre, both the small and large intestine have been used to replace the oesophagus [31, 32]. Long-term follow-up in replacement oesophagoplasties using the small intestine showed no negative consequences of reflux. Therefore, oesophagoplasty techniques using the jejunum and ileum have been developed and preferred over techniques combining the use of the small intestine and the large intestine together or the exclusive use of the large intestine [31, 32]. The Wrocław Centre reports that the best replacement organ for the oesophagus is the jejunum, followed by the ileum. However, using the jejunum for reconstruction of the entire oesophagus is only possible in 30–40% of patients due to the arrangement of mesenteric vascular arcades [32]. Bernat described a two-stage reconstructive technique with conduit conditioning of the jejunum [31]. In the first stage, the jejunum, pedunculated to the 4th mesenteric artery, is placed in a pre-sternal subcutaneous tunnel [31]. In the second stage, 4–5 weeks later, the intestine is mobilised from the subcutaneous bed. During the conditioning period, adaptive dilatation of the artery in the critical middle section of the arcade should allow for safe lengthening of the conduit of approximately 6–8 cm. The intestine can then be moved retrosternally and anastomosed with the oesophagus at the neck [31]. Jezioro’s original method involved creating a replacement oesophagus from the ileum with the caecum based on the vascularisation from the ileocecal artery. This was a difficult method due to the mesenteric vessel system and the presence of numerous lymph nodes in this area [32]. The development of microsurgical techniques has made it possible to safely replace the entire oesophagus with a long pedunculated segment of jejunum with microvascular anastomosis of the upper pedicle. Short stenosis of the cervical segment of the oesophagus, on the other hand, can be replaced with a free intestinal graft [27, 33].
Surgical techniques
A review of the literature does not provide a clear answer to the question of which organ constitutes the optimal replacement oesophagus [1, 2, 19, 24, 25, 29, 30, 33]. A selective approach to oesophageal resection is suggested, taking into account the age of the patient and the location of the stricture. Oesophagectomy is the preferred surgical procedure, although, in some patients, the location of the obstruction may justify a bypass procedure [23, 24]. In general, the aim is to remove the damaged oesophagus, and the location of the stricture will determine the type of resection and reconstruction.
Oesophagogastroplasty
Many authors believe that the stomach is the preferred organ for performing oesophageal replacement [30]. This view is supported by:
consistent, good and predictable vascularisation,
leaving a reservoir for food in case of whole stomach displacement,
single anastomosis,
relative ease of the procedure, with shorter operation time compared to other methods.
The reported mortality of oesophageal reconstruction using the stomach ranges from 0 to 10% [25, 34]. In Orringer’s series, anastomotic leakage was observed in 9% of patients [34]. Stenosis at the oesophagogastric anastomosis is a frequent complication, occurring in 8–46% of cases [25, 34]. Postoperative complaints, such as vomiting, regurgitation, and a feeling of fullness after meals, tend to resolve after a follow-up period of approximately nine months [25, 34].
Oesophagocoloplasty
The large intestine has traditionally been considered the optimal organ for oesophageal replacement [2, 19, 24, 28, 29]. However, surgical techniques can be more complex and time-consuming, and the risk of early complications such as graft ischaemia and necrosis, anastomotic leakage, early-onset anastomotic stenosis, gastrointestinal obstruction, respiratory failure, and surgical site infection ranges from 35% to 56% [19, 24, 25, 35]. When planning surgery in patients over 35 years of age, primary bowel diseases (e.g. polyps, bowel cancer) should be excluded by performing a colonoscopy. The most dangerous complication of oesophagocoloplasty is graft necrosis; therefore, careful assessment and selection of the arterial supply of the intestinal graft, and atraumatic surgical technique to prevent pressure on the vascular pedicle, are key considerations. Wain et al. advocate routine mesenteric arteriography, which may show deviations from typical vascular anatomy in 38% of patients [36]. Popovici also performed arteriography in all operated patients [29]. Other authors prefer transillumination of the mesentery [24, 28, 35]. The intestinal graft can be placed retrosternally, or in the posterior mediastinum. However, when choosing the retrosternal route, care should be taken to make the opening wide enough to pass the graft through the diaphragm. Some authors routinely excise the manubrium and a portion of the left clavicle and, if necessary, resect the lateral segment of the liver to eliminate any sites of potential compression which may lead to ischaemia of the bowel [35, 36]. The choice between the right and left half of the colon, aside from the characteristics of the bowel itself, should be based mainly on which has the most favourable vasculature. The left colon is characterised by a more constant course of the marginal artery of Drummond, which is absent in no more than 5% of patients [28]. Popovici’s approach to the selection of bowel segment uses the distal segment of the ileum as well as long segments of the colon up to and including the rectum [29] (Table V).
Table V
Properties of intestinal segments
| Segment | Adequate diameter | Peristalsis | Consistency of vascularisation | Anti-reflux mechanism |
|---|---|---|---|---|
| Right colon | +/– | +/– | – | – |
| Left colon | + | + | + | – |
| Ileocaecal segment | ++ | ++ | +/- | + |
The final decision on which bowel segment to choose for oesophageal reconstruction is made intraoperatively, after trial vessel closure, and before their planned ligation. According to the literature, resection of the oesophagus and its replacement with the large intestine is characterised by a high (35–56%) rate of early complications [19, 24, 25, 28, 35]. Thomas et al. divided significant early complications into groups of general and surgical complications [28].
General complications:
Surgical complications:
Late complications of oesophagocoloplasty:
stricture of oesophagocolonic anastomosis, diffuse graft stenosis,
reflux or heartburn, regurgitation, aspiration pneumonia,
ulceration of the graft,
chronic diarrhoea,
hernia of the graft to the pleura,
redundancy (too long, folded bowel),
paralysis of the recurrent laryngeal nerve,
gastrointestinal obstruction,
scar hernia,
The few studies on the long-term outcomes of reconstruction using the colon focus on outcomes of surgery for other indications such as oesophageal cancer and achalasia [28, 36]. A report by Chirica et al. [19] presented a large homogeneous group of patients in which long-term follow-up revealed the diagnosis of late intestinal conduit dysfunction in more than half of the operated patients, most of whom required surgical intervention. Usually surgical outcomes are characterised by an acceptable perioperative mortality rate. In this case, however, a high rate of early complications, including anastomotic leaks and postoperative strictures (Table VI), has also been observed.
Table VI
Outcomes of reconstruction of the oesophagus using the colon
| Author | Number of operated patients | Mortality (%) | Early complications (%) | Anastomotic leakage (%) | Late complications (%) | Anastomotic stenosis (%) | Reflux (%) | Redundancy (%) | Good/ satisfactory outcome (%) |
|---|---|---|---|---|---|---|---|---|---|
| Chirica et al. [19] | 223 | – | 56 | 15 | 55 | 29 | 11 | 5 | 77 |
| Popovici [29] | 347 | 4.6 | – | 6.9 | – | 6.3 | – | 0.3 | 93 |
| Wain et al. [36] | 20 | – | – | – | – | 48 | 8 | 4 | 80 |
| Boukerrouche [35] | 60 | 3.3 | 45 | 16.6 | – | 8.3 | 3.3 | 3.3 | 100 |
| Gerzic et al. [24] | 176 | 5.68 | 34.09 | 11.36 | 18.18 | 7.38 | – | 2.84 | 98 |
| Zhou et al. [25] | 71 | 7 | 35 | 23 | – | 8.5 | – | – | – |
| Włodarczyk et al. [37] | 23 | 0 | 34.7 | 17.4 | 4.3 | 4.3 | – | – | 85.7 |
Reconstruction of the oesophagus using the small intestine
The small intestine seems an ideal substitute for the oesophagus due to its matched diameter, lively peristalsis and resistance to reflux. However, its use is often not feasible. The Roux technique makes it possible to mobilise a segment of jejunum approximately 30 cm long, which can replace the distal part of the oesophagus. The Wrocław centre suggests that the best substitute for the oesophagus is the jejunum, followed by the ileum or the final 20 cm segment of the ileum together with the ascending colon [32]. Using the jejunum for the reconstruction of the entire oesophagus is only possible in 30–40% of patients due to the course of the mesenteric vascular arcades [32]. Bernat described the aforementioned technique of a two-stage reconstructive procedure with conditioning of the jejunum conduit, enabling lengthening of up to 8 cm [31]. The development of microsurgical techniques has made it possible to safely replace the entire oesophagus with a pediculed, long segment of jejunum with microvascular anastomosis of the upper pedicle. Short strictures of the cervical segment of the oesophagus can be replaced with a free intestinal graft [33]. The primary limitation of small bowel oesophageal reconstruction is the requirement for specific microsurgical operative techniques.
Summary
Chemical burn of the upper gastrointestinal tract is a difficult clinical problem both in the early and late stages. Reconstructive surgery of the oesophagus is characterised by a high rate of complications, and therefore patients require close postoperative surveillance. All patients with oesophageal burns, including those undergoing surgery or endoscopic treatment, require long-term follow-up due to the increased risk of cancer development in the burn scar and anastomotic area.