Summary
In our study, longer chronic total occlusion (CTO) duration was associated with longer procedure time, greater volume of dye used and lower revascularization success. However, it did not influence the in-hospital adverse event rate. This should be taken into account when planning procedures of CTO older than 12 months. Moreover, in our study longer CTO duration was associated with lower procedural success.
Introduction
Chronic total occlusion (CTO) is present in about 16% to 30% of patients undergoing coronary angiography [1–3]. Despite the increased experience due to the growth of the number of the performed procedures and the introduction of new techniques and equipment, CTO still remains one of the most demanding procedures in interventional cardiology. Using large registry databases, differences in outcomes and revascularization success in patients with CTO were published during the past years [4–9]. However, as determining age of the CTO may be very challenging, most of them did not include this factor in the analysis. There are several reports describing how and whether CTO duration may affect lesion and procedural characteristics [10–14].
However, none of those studies focused on such a large cohort of patients treated in recent years, and therefore benefiting the most from the latest developments in the field of CTO.
Material and methods
The European Registry of CTOs (ERCTO) is a prospective real-world monitored based registry involving over 100 centers across Europe including patients treated with CTO percutanous coronary intervention (PCI) [6].
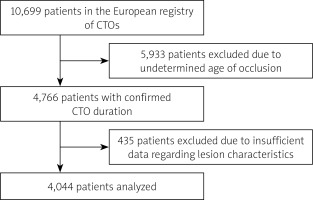
For the purpose of our study we included patients admitted to the hospitals between January 2015 and the end of April 2017. The treatment indication for CTO was symptomatic myocardial ischemia and/or evidence of reversible myocardial ischemia by perfusion imaging or stress testing. Registry data from both members and associates of the Euro CTO Club were included. Only patients with certain or likely CTO duration were included in the analysis. Out of 10699 patients in the database recruited during the selected period of time, 5933 patients were excluded due to undetermined age of the occlusion. Out of the remaining 4766 patients we excluded 287 patients with an additional acute coronary syndrome and 435 patients with insufficient further data regarding the occlusion characteristics. In the end, a total of 4044 patients were included in the data analysis (Figure 1). Patients were divided into 3 groups according to age of the CTO: 1) 3–6 months; 2) 7–12 months; 3) over 12 months.
Figure 1
Flow chart. Out of 10699 patients in the database recruited during the selected period of time, 5933 patients were excluded due to undetermined age of the occlusion. Out of the remaining 4766 patients we excluded 287 patients with an additional acute coronary syndrome and 435 patients with insufficient further data regarding the occlusion characteristics. In the end a total of 4044 patients were included in the data analysis
Coronary CTO was defined as angiographic evidence of total occlusions with thrombolysis in myocardial infarction (TIMI) flow grade of 0 and estimated duration of at least 3 months. The length of coronary occlusions was estimated from angiographic projections. Degree of calcification was estimated visually on fluoroscopy. Moderate and severe calcifications were defined as calcium extending for less than half or equal/greater than half of the total CTO segment, respectively. The assessment of collateral connections was made according to the Werner classification (CC) [15]. Occlusion duration in the ERCTO was divided into 3 levels of certainty (certain and angiographically confirmed; likely and clinically confirmed; undetermined), as suggested by the Euro CTO Club consensus document [16]. Procedural success was defined as angiographic success (final residual stenosis < 30% by visual estimation and TIMI flow grade of 3 after CTO recanalization). In-hospital adverse events (AEs) were defined as the composite of non-Q-wave and Q-wave myocardial infarction (MI), coronary perforation requiring intervention, recurrent angina requiring urgent repeat revascularization with PCI or coronary bypass surgery, major bleeding, stent thrombosis, stroke, and death. The complexity of CTO lesion was assessed through the J-CTO (Multicenter CTO Registry in Japan) score and the clinical and lesion-related (CL) score [17, 18].
Statistical analysis
Categorical variables are presented as counts and percentages (%). The median (25th–75th percentiles) is reported for continuous data. Fisher’s exact test or c2 test was used for categorical variables, and the Mann-Whitney U test was used to compare continuous variables. Multivariable logistic regression analyses were performed to determine the independent predictors for lesion and procedural characteristics. Univariate analysis was performed for all variables in the study, then the variables with p < 0.05 were included in the multivariable models for adjusted analysis. Statistical analyses were performed with R 3.4.
Results
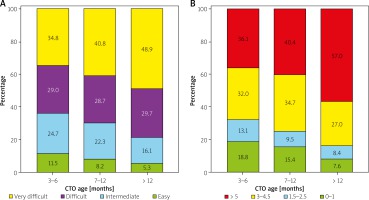
A total of 4044 of patients were included in the main analysis. Patients with the longest CTO duration as compared to patients with CTO duration 3–6 months were significantly older (63.0 (56.0–70.0) vs. 66.0 (59.0–73.0) years) and had higher prevalence of peripheral artery disease (8.8% vs. 12.4%) (Table I). The group with the longest CTO duration had higher incidence of myocardial infarction (MI) and prior coronary artery bypass grafting (CABG) as compared to other groups. Lesion and procedural characteristics also differed between patients with different age of the CTO (Tables II, III). Patients with the longest CTO duration as compared to patients with CTO duration 3–6 months had longer occlusions (28.0 (20.0–40.0) vs. 20.0 (15.0–30.0) mm), more calcified lesions (calcification moderate or severe; 51.6% vs. 34.4%), better collateral circulation (30.1% vs. 24.7% for CC2) and more advanced coronary artery disease distal to CTO (48.8% vs. 34.5%). The revascularization success rate was the highest in the patients with CTO duration 3–6 months as compared to the patients with the oldest CTO (92.1% vs. 83.5%), and the retrograde approach was less common in those lesions (24.2% vs. 38.5%). Intravascular ultrasound was used more frequently in the older occlusions (15.7% vs. 9.5%). Incidence of in-hospital AEs increased from 2.0% in the group with the shortest CTO duration to 3.6% in the group with the longest CTO duration (Table III). Time of the procedure and dye volume increased significantly with increased CTO duration, reaching up to 120 min and 250 ml of dye used in the last group (Table III). The J-CTO score as well as the CL score were higher in older lesions (Figure 2).
Table I
Clinical characteristics of patients depending on the chronic total occlusion age
Table II
Lesion characteristics depending on the chronic total occlusion age
Table III
Procedural characteristics depending on the chronic total occlusion age
Figure 2
Differences in J-CTO score (A) and CL score (B) depending on the chronic total occlusion age. Differences in J-CTO score between patients with different chronic total occlusion (CTO) duration were significant (3968 patients; p < 0.001). J-CTO score of 3 or more increased with increase of the lesion age. In the CL score only patients with a first attempt were included in the analysis (2692 patients). Differences in CL score depending on the occlusion duration were significant (p < 0.001) and prevalence of a CL score of more than 5 points increased with the lesion age
In multivariate analysis (univariate analysis is presented in Table IV) we found that CTO duration was an independent predictor of lesion length, severity of calcification, better developed collateral circulation and procedure failure, but it did not influence the AE rate (Table V). Full multivariate analysis is presented in Table VI.
Table IV
Univariate analysis for lesion characteristics
[i] Values are median (25th – 75th percentile) or n (%). AE – adverse events, BMI – body mass index, CABG – coronary artery bypass grafting, COPD – chronic obstructive pulmonary disease, CTO – chronic total occlusion, Cx – circumflex artery, EF – ejection fraction, GFR – glomerular filtration rate, LAD – left anterior descending artery, LM – left main artery, MI – myocardial infarction, PAD – peripheral artery disease, PCI – percutaneous coronary intervention, RCA – right coronary artery. For calcification, lesion length and collateral circulation only clinical predictors were included in the analysis. For procedural success and AE clinical, lesion characteristics and retrograde approach were included in the analysis.
Table V
Predictors for lesion characteristics and adverse events – multivariate analysis
[i] Only results for occlusion duration (as compared to occlusion duration 3–6 months) are presented in the table. Full multivariate analysis is presented in Table VI. AE – adverse events, CI – confidence interval, CTO – chronic total occlusion, OR – odds ratio. Number of patients included in the analysis (n): calcification (moderate or severe), n = 3589; collateral circulation type 2, n = 3739; Lesion length 20 mm, n = 3815; AE, n = 4037.
Table VI
Full multivariate analysis
[i] AE – adverse events, BMI – body mass index, CABG – coronary artery bypass grafting, CAD – coronary artery disease, CI – confidence interval, CKD – chronic kidney disease, COPD – chronic obstructive pulmonary disease, CTO – chronic total occlusion, Cx – circumflex artery, EF – ejection fraction, LAD – left anterior descending artery, LM – left main artery, OR – odds ratio, PAD – peripheral artery disease, PCI – percutaneous coronary intervention. 1As compared to occlusion duration 3–6 months; 2as compared to right coronary artery. For calcification, lesion length and collateral circulation only clinical predictors were included in the analysis. For procedural success and AE clinical, lesion characteristics and retrograde approach were included in the analysis. Number of patients included in the analysis (n): calcification (moderate or severe), n = 3589; collateral circulation type 2, n = 3739; lesion length 20 mm, n = 3815; procedural success, n = 3513; AE, n = 4037.
Differences between patients with undetermined age of the occlusion and known occlusion duration are presented in the Supplement (Table VII). As expected, patients with determined age of occlusion had previous MI and CABG more frequently (37.5% vs. 44.8% and 8.1% vs. 17.3% respectively). The procedural failure rate was similar in both groups (11.9% vs. 12.2%). Interestingly, incidence of AEs was higher in the group with undetermined age of the occlusion (3.7% vs. 2.8%).
Table VII
Comparison between patients with undetermined age of the occlusion and known occlusion duration
[i] Values are median (25th–75th percentile) or n (%). AE – adverse events, BMI – body mass index, CABG – coronary artery bypass grafting, COPD – chronic obstructive pulmonary disease, CTO – chronic total occlusion, Cx – circumflex artery, EF – ejection fraction, GFR – glomerular filtration rate, LAD – left anterior descending artery, LM – left main artery, MI – myocardial infarction, PAD – peripheral artery disease, PCI – percutaneous coronary intervention, RCA – right coronary artery. Out of 5933 patients with undetermined age of the occlusion, 900 were excluded due to insufficient data regarding the occlusion characteristics. In the end a total of 4044 patients with known occlusion duration and 5033 patients with undetermined age of the occlusion were included in the data analysis.
Discussion
Our study, performed on a large cohort of patients, demonstrated that: 1) longer duration of CTO is associated with longer procedure and fluoroscopy time, and greater amount of dye used; 2) longer duration of CTO is a predictor of greater calcification, longer lesions and more developed collateral circulation; 3) age of the CTO influences the procedure success, but not in-hospital AEs.
Procedure duration
As observed in previous studies, patients with longer CTO duration had longer procedure and fluoroscopy time, as well as a greater amount of dye used [10]. These results should be taken into account when planning a procedure with known age of the CTO.
Calcification
In our study occlusion duration of over 12 months was an independent predictor of calcification. In more advanced coronary artery disease (CAD), elevated lipid content and inflammatory mediators induce osteogenic differentiation of vascular smooth muscle cells in the intima – usually calcific deposits are found more frequently and in greater amounts among more advanced lesions and in elderly individuals [19]. These observations were confirmed in the CTO histopathology study where older lesion age was associated with a greater fibrocalcific component [20]. Comparable to our results, Danek et al. recently observed a difference of 16% in moderate/severe calcification between patients with a CTO duration shorter than 5 and longer than 36.3 months [10]. Moreover, as previously reported, CABG in medical history was also associated with higher prevalence of calcification in CTO lesions [21]. Importantly, calcification is regarded as one of the predictors of failure when performing PCI of CTO – both the CL score proposed by Alessandrino et al. and the J-CTO score include calcification in their scoring systems [17, 18].
Collateral circulation
We observed that occlusion duration longer than 6 months was a predictor of development of collaterals in CC grade 2 proposed by Werner et al. [15]. Time of recruitment of collaterals in CTO is still debatable, ranging from several weeks to months [22]. Collateral flow plays several major roles in CTO. First, well-developed collaterals have the capacity to prevent myocardial necrosis and may preserve myocardial viability [22]. Second, collaterals are used during the retrograde approach and thus poorly developed collaterals were found to be an independent predictor of technical failure in CTO PCI [5, 23].
Lesion length
In contrast to the study of Danek et al., we found that CTO length increased with age of the CTO [10]. In our study, even occlusion duration longer than 6 months was an independent predictor of CTO length over 20 mm. One could speculate that with the novel techniques including the hybrid approach and retrograde revascularization lesion length may be less important in assessing complexity of the CTO procedures as shown in the PROGRESS CTO score and ORA score [5, 23]. In contrast, Ellis et al. showed in their recent study assessing predictors of a successful hybrid approach that procedure failure is correlated with occlusion length over 10 mm [7]. Besides that, longer lesions may still influence the duration of the procedure [17].
Procedural success
Currently several different angiographic scoring systems assessing predictors of failure and success in CTO PCI are available, but none of them has ever included the duration of CTO in their analysis [24–26]. On the other hand, CTO duration as predictor of revascularization failure has been described in computed tomography studies [13, 14]. Given the results of those studies and our current study, it is plausible to say that duration of CTO may have an influence on procedural success and PCI of CTO should not be postponed, although this observation merits further research. Nevertheless, it should be underlined that in our study the exact age of CTO lesions could only be determined in less than 50% of the cases; thus implementing CTO duration in future scoring systems may be challenging. Lastly, some of the studies did not find a correlation between CTO duration and procedural success; however, the number of patients included in the analysis was also significantly lower [27].
Adverse events
A weighted meta-analysis by Patel et al. with 18061 patients included from 65 studies revealed low rates of AEs in patients undergoing CTO PCI [2]. In our study rates of AEs were comparable, with 0.4% deaths and 0.7% MI during the hospitalization period. A recent study showed that complications during PCI of CTO were more frequent in females [28]. In concordance with that observation, female gender was one of two AE predictors in our study. Not surprisingly, the retrograde approach was the other predictor of AEs as this approach is considered as more complex when compared to the antegrade approach [6, 29]. However, it should be noted that the retrograde approach is often used in very advanced lesions where the antegrade approach is not feasible or ended with failure. Importantly, although patients with the longest CTO duration had higher incidence of AEs as compared to the patients with the shortest CTO duration, the age of the CTO was not an independent predictor of AEs. Barlis et al. in their study compared AEs in patients with undetermined and known occlusion duration [30]. In long-term follow-up they found that undetermined occlusion duration was a predictor of AEs. In contrast to our study they did not find any differences in in-hospital outcomes between groups with known and unknown occlusion duration. However, their study was limited by the sample size.
Limitations
First, our study is limited by its observational design. Second, angiography-dependent and clinical outcomes were not independently adjudicated. Third, data regarding patients and lesion and procedural characteristics were missing in some cases. Moreover, only in half of the patients could CTO age be assessed. Out of 10 699 patients, 4044 (37.8%) were included in the final analysis, which could have involved selection bias. Further, patients excluded from the study differed from those included in important lesion characteristics such as lesion location, number of previous attempts and severity of coronary artery disease. Lastly, the exact age of the CTO is often unclear. Hence, it is often very challenging to determine the exact age of the CTO.
Conclusions
Longer CTO duration is associated with greater prevalence of calcification, longer lesions, and better developed collateral circulation. Most importantly, in our study longer CTO duration was associated with lower revascularization success by PCI. However, it did not affect the rate of in-hospital AEs. Our results should be taken into account when planning procedures of CTO older than 12 months.








