A patient with peripheral artery disease (PAD) was selected randomly from a group of 55 patients from the N-O CLI clinical trial, registered under EudraCT No. 2016-004684-40, conducted in accordance with GCP requirements. A 76-year-old woman was referred to the Outpatient Clinic of the Vascular Surgery Department of the John Paul II Hospital in Krakow, due to critical ischemia of the right lower limb. She was offered an experimental method of treatment using mesenchymal stem cells (MSCs). The patient was submitted to magnetic resonance imaging (MRI) examination before (E1) and after (E2) the intraarterial and intramuscular injections of CardioCell based on mesenchymal stem cells (MSCs) or placebo which was produced according to the rules of good manufacturing practices at Jagiellonian University Collegium Medicum, Pracownia-Bank Tkanek i Komórek (PBTiK) Zakładu Transplantologii UJ CM (license no. 145/0323/15), in a double-blind RCT. Therapy encompassed three injections every 7 weeks and after 398 days after E1 the condition of the lower extremities was once again checked by MRI. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of John Paul II hospital in Krakow. Positive opinion no. 1072.61201.17.2017 dated on 21/12/2017 was issued by the Bioethics Committee of the Jagiellonian University.
Muscles’ condition was evaluated based on T1- (T1WI) and T2-weigthed images (T2WI; Figure 1), obtained transversely on a 3T MR system (Siemens Skyra 3 T, Erlangen, Germany) using an eight-channel TORSO body coil with the application of turbo spin echo (TSE) and turbo inversion recovery magnitude (TIRM) with fat suppression pulse sequences, respectively. Complementarily, diffusion tensor imaging (DTI) was performed using the Echo Planar Imaging (EPI) sequence with six diffusion gradient directions. In the DTI protocol, b-value = 350 s/mm2, number of scans, NoS = 1, while slice thickness was equal to 8 mm. The BSD-DTI calibration method [1, 2] was applied before the quantitative analysis, for which it was shown recently to have substantial applicability [3]. The following DTI parameters were analyzed: the second and third eigenvalues (λ2 and λ3, respectively), mean (MD), longitudinal (DL) and transversal (DT; mean of λ2 and λ3) diffusivity, and fractional anisotropy (FA), by using the in-house BSD-DTI software (BSD-DTI ver. 2.0, AGH UST, Krakow, Poland). For comparison, mean parameters for 3 healthy volunteers were presented (control, C). A single muscle from each compartment was chosen as representative: tibialis anterior (TA), soleus (SOL) and gastrocnemius medialis (GM).
Figure 1
Axial T1-weighted FSE (T1WI) and T2-weighted TIRM (T2WI) images of calf of a 76-year-old woman with PAD. A, B – T1WI (A) and T2WI (B) before the medical intervention (examination E1); C, D – T1WI (C) and T2WI (D) after the injections of CardioCell based on mesenchymal stem cells (MSCs) or placebo in a double-blind RCT (examination E2). Regions of fatty infiltration (arrows) and edema-like signal (arrowheads) can be recognized. In the picture, analyzed calf muscles are indicated by the following abbreviations: tibialis anterior (TA), soleus (SOL), gastrocnemius medialis (GM), for which T1 and T2 signal intensities (T1SI and T2SI, respectively) are shown below the image
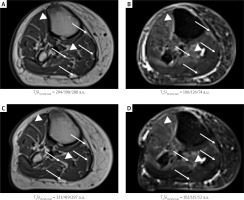
Recently it was shown that the BSD-DTI method is crucial for the quantitative evaluation of the muscle condition and intervention effect in PAD. In this study we found that diffusion can also reflect different disease courses. MRI of the patient from this work revealed chronic muscle denervation that resulted in atrophy with fatty infiltration reflected in a hyperintense signal on T1WI and a corresponding low-intensity signal on T2WI [4], similarly to a previously reported patient [3]. In contrast, anterior and deep posterior compartments showed a high-intensity, edema-like signal on T2WI (Figure 1), while neoangiogenesis in the muscle bulk was hardly recognized, which is opposite to the previously reported patient [3].
According to Mazur et al. [3], each muscle change in PAD can be reflected in diffusive properties. λ2 and λ3 reflect the transverse diffusion in the endomysium and myofiber, respectively. Hence, λ3 is sensitive to fatty infiltration, which relies on the replacement of muscle fibers by fat characterized by a smaller diffusion coefficient than water. Increased diffusivities in general, but especially DT, λ2 and λ3, can indicate edema. Interestingly, the patient did not demonstrate increased diffusivities in E1. Visibly lower DT resulting from the lower λ3 was observed for SOL, suggesting the highest fatty deposit. In TA the highest DT, λ2, λ3 and MD are correlated with the strongest edema. GM muscle condition was not severe based on T1WI/T2WI, and among the three analyzed muscles it had only slightly increased diffusivities.
After the intervention (E2) all muscles are characterized by higher T1 signal intensity (T1SI) and lower T2 signal intensity (T2SI) (Figures 1 C and D, respectively), which is associated with T1 and T2 decreasing. This means that they approached the values reported for normal muscles (T2 = 27–38 ms [5] and T1 = 1420 ms [6] in comparison to T2 = 133 ms for fat [6]). At the same time, MD and DT decreased, which suggests the reduction of the edema. Moreover, GM and TA showed improved muscle anisotropy and FA, visible also as the improved structure (density and direction of fibers) on the tractographic DTI representation (Figures 2 B and C). A slight decrease of λ2 and λ3 in TA and GM seems to be connected with edema recovery rather than fatty degeneration. In SOL, apart from decreased DT, λ2, λ3 and MD indicatory for detumescence, fiber tract density (Figures 2 B and C, arrowheads), FA and DL decreases can also be observed, which suggests overall muscle dehydration. The intervention caused changes from peripheral to internal compartments, which will be assigned to the MSC therapy or placebo after the unblinding.
Figure 2
Diffusion tensor imaging (DTI) of calf muscles of a 76-year-old woman with PAD. A – DTI parameters (fractional anisotropy, FA, mean diffusivity, MD, longitudinal diffusivity, DL, transverse diffusivity, DT, the second eigenvalue, λ2, and the third eigenvalue, λ3) obtained before (E1) and after (E2) the intervention consisting of the injections of CardioCell based on mesenchymal stem cells (MSCs) or placebo in a double-blind RCT, with comparison to the values obtained for healthy control (C) and the patient in Ref. 2 [2] B, C – DTI tractography for calf muscles for a given region of interest in E1 (B) and E2 (C). Regions of improved fiber tract direction (arrows) and density (arrowheads) can be recognized. In the picture, analyzed calf muscles are indicated by the following abbreviations: tibialis anterior (TA), soleus (SOL), gastrocnemius medialis (GM)

In conclusion, without DTI no information about muscle structure can be obtained from T1WI/T2WI. As a reference we showed results from the previous study, in which T1WI/T2WI indicated more collateral circulation and fatty replacement in the patient’s calves, suggesting more disrupted muscles than for the patient in this study. The same intervention procedure caused more subtle changes towards the healthy control’s diffusivities and anisotropy, meaning that despite advanced lower limb ischemia, collateral circulation, even though intruding the muscle bulk, allowed the muscle structure to be preserved and the muscles to remain nourished. Surprisingly, the patient in this study was found to have more damaged muscle structure in SOL, while demonstrating a great improvement in anterior and posterior compartments after the intervention.








