Introduction
Hepatitis C virus (HCV) infection is a worldwide health concern. Since chronic HCV infection may progress to advanced liver disease, cirrhosis and hepatocellular carcinoma (HCC), HCV-related mortality is estimated at about 500,000 deaths each year [1].
Hepatocellular carcinoma is highly correlated with liver fibrosis degree, and develops in 1-7% of cirrhotic patients [2, 3]. HCV eradication can prevent the development of high stage liver fibrosis and decrease associated mortality [4, 5]. HCV is also associated with extrahepatic manifestations, including cryoglobulinemia, porphyria cutanea tarda, diabetes mellitus, neuropsychiatric disorders [6], cognitive impairment, processing speed and concentration [7-9].
As a result of the effect of HCV on the liver as well as on other organ systems (extrahepatic manifestations of HCV), HCV has been widely reported to have a profound negative impact on health-related quality of life (HRQL), work productivity and other patient-reported outcomes [10].
In previous years, it has been shown that interferon IFN-based successful treatment, i.e. sustained viral response (SVR), correlated with better prognosis [11]. However, since the introduction of direct antiviral agents (DAAs), which are more tolerated and effective than INF, some concerns have been raised considering higher HCC incidence in these patients [12, 13], although the ANRS collaborative study group examined three large French prospective multicenter studies of HCV patients treated with DAAs and found no increased risk of HCC in patients treated with DAAs [14]. However, INF-based treatment had many side effects and was often discontinued because of these side effects. During treatment with INF, quality of life was seriously impaired [11].
The effect of treatment with DAAs on quality of life has been studied recently, with conflicting results. While Younossi et al. found that treatment with DAAs correlated with improvement in quality of life regardless of the fibrosis degree [10], Kawakubo et al. concluded that the quality of life did not change after similar treatment [15].
This study aims to discuss the long-term quality of life after DAA treatment as compared to baseline.
Material and methods
A study was conducted at Rambam Health Care Campus in Haifa, Israel, between January 2015 and August 2018. Every third patient was randomly assigned to the study. Background data, including HCV-related data, were gathered from the digital profiles both before treatment and six months after the treatment. The Model for End-stage Liver Disease (MELD) score was calculated for each patient. Glomerular filtration rate (GFR) was calculated by the modification of the diet in renal disease (MDRD) equation.
Quality of life was estimated by the Translating and Testing the Liver Disease Symptom Index II (LDSI 2.0) score (Appendix), which includes nine symptoms: itch, joint pain, right upper quadrant pain, sleepiness during the day, family worry, decreased appetite, depression, fear of complications, and jaundice. The severity of each symptom may vary from 0 (not at all) to 5 (very severe). This score is highly validated for severity of liver disease symptoms [16]. The study staff called every patient by telephone to verify the severity of symptoms before and after treatment.
The primary end point was the long-term effect of DAAs on the nine symptoms listed above. The secondary endpoints were the effect of DAAs on the average of LDSI 2.0 symptom scores in men and women, and in different fibrosis stages.
The study was approved by the local institutional review board. All the authors had access to the data and have reviewed and approved the final manuscript.
Statistical analysis
Processing and analysis of the statistical data was done with IBM SPSS statistical software, version 24. For continuous variables, the mean, median and standard deviations were calculated. The t-test and Mann-Whitney test were used to examine the differences between the continuous variables in the investigation groups. To investigate the categorical parameters, the χ2 test or Fisher’s exact test was used. All the statistical tests were two-sided, with a p value of ≤ 0.05 considered as statistically significant. Data analysis was done after the end of data collection.
Results
One hundred patients were included in the study, which was conducted over a 30-month period. Background data are summarized in Table 1.
Table 1
Background data, before treatment was started
[i] SD – standard deviation, HIV – human immunodeficiency virus, HBV – hepatitis B virus, INR – international normalized ratio, GGT – γ-glutamyltransferase, ALP – alkaline phosphatase, ALT – alanine aminotransferase, AST – aspartate aminotransferase, LDL – low-density lipoprotein, HDL – high-density lipoprotein, TRIG – triglycerides
The vast majority (84%) of the patients suffered from a high degree of fibrosis (F3/F4) according to the METAVIR score. The number (and percentage) of patients with F0 + F1, F2, F3 and F4 fibrosis degree was 6 (6%), 10 (10%), 42 (42%) and 42 (42%) respectively. The most common genotype was G1B (84 (84%) patients; the prevalence of the genotypes 1A, 2, 3 and 4 was 4 (4%), 1 (1%), 10 (10%) and 1 (1%) respectively. Three patients suffered from ascites (3%), 16 from esophageal varices (16%) and one from hepatic encephalopathy (1%).
Sixty patients (60%) were treated with paritaprevir, ombitasvir, ritonavir ± dasabuvir, one (1%) with simeprevir, sofosbuvir, 12 (12%) with each of ledipasvir, sofosbuvir, and daclatasvir, sofosbuvir, nine (9%) with elbasvir, grazoprevir and six (6%) with sofosbuvir, velpatasvir. Nine patients (9%) received 24-week therapy, and the rest were treated for 12 weeks. None of the patients had previous major mental illness, while 13 were previous intravenous drug users.
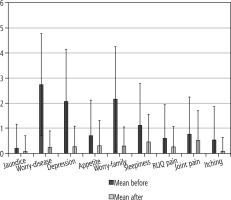
Regarding quality of life, significant improvement was seen in 7/9 parameters, including itching, pain in the right upper abdomen, sleepiness during the day, worry about family situation, decreased appetite, depression and worry about liver disease complication. However, joint pain and jaundice did not change significantly after treatment (Fig. 1).
Fig. 1
Mean and standard deviation of symptoms before and after treatment
SD – standard deviation, RUQ – right upper quadrant, Sleepiness – day-time sleepiness, Worry-family – worry about family situation, Appetite – decreased appetite, Worry-disease –worry about liver disease
The mean value of the nine questions, which may be used as a summary of quality of life, was 1.22 ±0.95 before treatment, median 1.1 (0-3.78); after treatment it was reduced to a mean of 0.29 ±0.47, median 0.11 (0-2.78), p = 0.001.
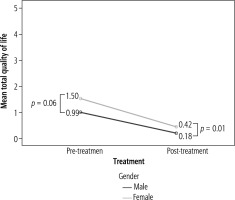
When subdividing the cohort by gender, men had a better quality of life than women, both before and after treatment. Quality of life improved after treatment in both gender groups (Fig. 2).
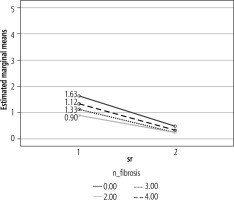
While assessing the quality of life in different fibrosis stages, life improved significantly after treatment in all fibrosis stages (Table 2, Fig. 3). All the fibrosis groups had a similar post-treatment quality of life.
Table 2
Mean LDSI II score in different fibrosis stages
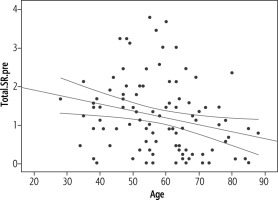
An inverse relationship was found between age and quality of life, which was statistically significant (p = 0.012) but weak (R = 0.252) (Fig. 4).
Some laboratory data changed after treatment. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) declined from 98 and 91 U/l, respectively, to 35 and 29 U/l, p < 0.001. Moreover, bilirubin and hemoglobin declined from 0.91 mg/dl and 13.8 g/dl to 0.75 mg/dl and 13.28 g/dl, while white blood cells rose from 6 to 6.79 × 103 cells/dl. On the other hand, GFR declined from 95.7 ml/min before treatment, to 91.4 ml/min after treatment. All these changes were statistically significant, but clinically insignificant. Platelets did not change significantly.
Discussion
There has been huge progress in the treatment of HCV in recent years. Treatment with DAAs is very effective and tolerated with minimal adverse events. In the past, the treatment of HCV included pegylated-interferon (PEG-IFN) and ribavirin (RBV). This treatment was very difficult for patients and caused multiple adverse events. During treatment, quality of life was impaired. On the other hand, after reaching SVR, the quality of life of these patients was improved compared to their status before reaching SVR [17-23]. To date, there are no studies which examined the effect of DAAs on the long-term quality of life of these patients.
Our study observed the long-term influence of DAA treatment in 100 patients who had finished treatment with DAAs at least 6 months before enrollment, and some were three years after completion of treatment. Mean time between the start of the study and the end of the antiviral therapy was over 29 months.
This study showed a significant improvement in the long-term quality of life after treatment with DAAs. In addition, statistically significant improvements were seen in some of the laboratory tests, as expected. We also found that the improvement in quality of life was not related to gender or fibrosis stage.
A number of studies have evaluated quality of life after treatment with DAAs in the short term. To the best of our knowledge, our study is the first to evaluate quality of life in the long term.
A previous study examined HRQL in 28 patients with chronic hepatitis C who received daclatasvir and asunaprevir for 24 weeks. The study showed that this combination improves quality of life in the short term. In addition, the nutritional status and liver fibrosis were improved in these patients and, therefore, the authors concluded that DAAs can induce long-term improvements in quality of life. Quality of life was assessed at three time points: before starting treatment, and at 12 and 24 weeks after completion of treatment [24].
Another cross-sectional study involved 147 Brazilian patients and compared quality of life with treatment with PEG-IFN and RBV vs. treatment with DAAs. Most (69%) received PEG-IFN and RBV dual therapy and the remaining patients received DAAs. During treatment, quality of life was impaired by IFN-based therapies due to a significant number of side effects and also due to injections of IFN. On the other hand, quality of life did not change in patients who received DAAs [25].
The effect of IFN-free treatments on quality of life in patients with chronic hepatitis C was examined in a study of 686 patients who received PEG-IFN, sofosbuvir (SOF) and RBV for 12 weeks (n = 155) or SOF + RBV for 12-24 weeks (n = 531). Eastern Asian HCV patients had significant impairment of HRQL during IFN treatment. Conversely, IFN removal from the treatment protocols significantly improved their quality of life [26].
In our study there was no significant improvement in joint pain in the patients after successful treatment. This finding is surprising because there is a significant association between joint pain and arthritis and chronic HCV infection. The sample size may not have been sufficient to estimate this point. Moreover, the severity of this symptom at baseline was low in our cohort.
Another finding in our study was deterioration of renal function after SVR. We expected an opposite result since there is a correlation between infection with HCV and kidney diseases. The patients in our study had normal renal function before beginning treatment with DAAs, and this may be the reason why we did not see improvement of renal function after SVR. This issue needs to be assessed in a larger group of patients, especially patients with renal impairment before treatment.
An improvement in quality of life in our cohort was seen in both genders. It is important to note that the quality of life of women with HCV was lower than that of men before treatment. Even after treatment, the improvement in quality of life in women did not reach a similar level to men. In addition, improvement in quality of life was found to be significant in patients with mild liver fibrosis as well as in patients with advanced fibrosis, including cirrhotic patients.
Our study has several limitations: this is a single- center, retrospective study. Patients were treated with different antiviral protocols. Moreover, the questionnaires were undertaken at a point of time but not on several occasions, so that the results might be influenced by acute events.
In conclusion, DAAs are very effective and well-tolerated drugs for treating chronic HCV patients and they significantly improved patients’ quality of life in the long term.