Introduction
In patients with lung cancer, the organs with the highest frequency of metastasis are the lung, bone, brain, liver, and adrenal gland [1–3]. Metastasis to the pancreas is extremely rare [4–6]. Although rare, pancreatic metastasis might cause obstruction of the biliary tract, limiting the administration of antitumour drugs that are metabolized in the liver and excreted from the biliary system [7–10]. With the widespread use of treatments such as biliary stents, restrictions on these anticancer drugs for lung cancer have been removed in a significant number of patients, and improvement in prognosis is expected [11, 12]. In patients with lung cancer, metastasis to the pancreas might occur during dissemination to multiple organs, but this is not always the case, and it seems that there are organs that frequently metastasize at the same time [4]. Our previous study was unable to analyse patterns of multi-organ metastasis [4]. Cluster analysis is a method of classifying things as patterns and has been a research method that has been frequently used in basic medical fields such as gene analysis and bacterial classification [12, 13]. We have recently conducted some studies on metastatic patterns using this method [14, 15].
The primary purpose of this study was to capture the metastasis in lung cancer patients with pancreatic metastasis as patterns by using cluster analysis. In addition, this study aimed at creating basic data that would lead to subsequent efficient examinations and treatments for patients with pancreatic metastasis of lung cancer.
Material and methods
Patients
Patients who presented with pathologically diagnosed lung cancer between April 2012 and March 2022 at our 3 tertiary hospitals were identified retrospectively via computerized searches of tumour registry data. Clinicopathological information such as age, gender, and histopathology in lung cancer were collected. Information from diagnostic imaging, including chest computed tomography (CT), brain magnetic resonance imaging or enhanced head CT, bone scan, ultrasonography and/or CT of the abdomen, and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), was used to identify the location of metastatic tumours. Information on distant metastases was collected in detail.
Pancreatic metastasis
Lung cancer pancreatic metastasis was diagnosed based on the following definitions. In patients with pathological specimens obtained from pancreatic metastases:
confirmation of SCLC,
confirmation of lung adenocarcinoma based on immunostaining results in adenocarcinoma.
Among patients for whom pathological specimens could not be obtained, we first selected patients who had no history of pancreatic disease. 18F-fluorodeoxyglucose uptake in pancreatic lesions was confirmed in patients who could perform FDG-PET. Then, based on images of pathologically-confirmed pancreatic metastasis from lung cancer [16–26], a diagnosis was made in the following conditions:
Statistical analysis
Cluster analysis was performed to classify patients [12, 13]. Briefly, pre-clusters to reduce the size of the matrix that contained the distances between all possible pairs of cases were performed. Then, the standard hierarchical clustering algorithm was applied to the pre-clusters to explore a range of solutions with different numbers of clusters. At this point, hierarchical cluster analysis was performed using Ward’s method to generate a dendrogram for estimation of the number of likely clusters within the population. Cluster boundaries were defined by large differences between successive fusion levels [12, 13]. At each cluster, samples were merged into larger clusters to minimize the within-cluster sum of squares or to maximize the between-cluster sum of squares in Euclidean distance. Variables for cluster analysis included the common metastatic sites described above. Statistical analyses were performed using BellCurve for Excel (version 3.0). Differences in proportions between 2 and among 3 independent groups were compared using the χ2 test. Overall survival (OS) was calculated with Kaplan-Meier analysis and compared using the log-rank test. P < 0.05 was considered statistically significant.
Results
During the study period, 1659 patients (1093 adenocarcinomas, 249 SCLCs, 232 squamous cell cancers, 71 large cell carcinomas, and 14 others) were diagnosed with lung cancer pathologically. Among them, 33 (2.0%) patients were diagnosed as having pancreatic metastasis at the time of diagnosis of lung cancer. Of them, one patient was diagnosed with SCLC by cytological examination of specimens collected by endoscopic ultrasound-guided fine-needle aspiration. The remaining 32 patients were diagnosed as having pancreatic metastasis from lung cancer by imaging. Positron emission tomography was performed in 7 cases, and all patients showed FDG uptake in pancreatic metastatic lesions. Shapes of the metastasis were solitary nodule in 29 patients, 2 nodules in 3 patients, and 3 nodules in one patient. The metastatic sites were 18 in the head, 14 in the body, and 6 in the tail of the pancreas. The median size of the metastatic nodules was 29 mm (range 10–49 mm).
Of the 33 patients, 28 (84.8%) were male. Eighteen, 14, and one patient had small cell lung cancer (SCLC), lung adenocarcinoma, and large cell neuroendocrine carcinoma, respectively. The median (range) age of SCLC patients was 67 (42–83) years and that of adenocarcinoma patients was 64 (44–80) years. There was no difference in age between these histological types (p = 0.955). All the 18 patients with SCLC were men, and 9 of the 14 patients with adenocarcinoma were men. There was a statistical difference in gender between the SCLC patients and those with adenocarcinoma (p = 0.010). There was no difference in the frequency of pancreatic metastasis between lung adenocarcinoma and squamous cell carcinoma (p = 0.148), but there was a significant difference in the frequency of pancreatic metastasis between SCLC and squamous cell carcinoma (p = 0.001).
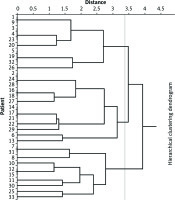
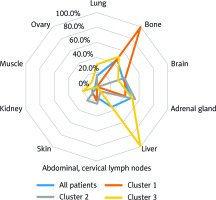
Figure 1 shows the dendrogram of 3 clusters created based on these metastatic sites in 33 patients with pancreatic metastasis. In this cluster model, metastatic groups were identified as follows: Cluster I (cluster characterized by bone metastasis, number of patients = 10), Cluster II (l cluster characterized by brain/adrenal metastases, number of patients = 13), and Cluster III (cluster characterized by liver metastasis, number of patients = 10). Demographic and baseline clinical and pathological characteristics of the identified clusters are shown in Table 1. There was no significant difference in age and histology among the 3 groups, but there was a significant difference in gender (p = 0.026, χ2 test). Figure 2 shows the frequency of metastatic sites. There was significant difference in frequency of the metastatic sites in these 3 clusters of patients (χ2 test, p = 0.001).
Table 1
Clinicopathological characteristics of lung cancer patients who were diagnosied as having pancreas metastasis
Median OS of our 32 patients was 4 months (range 1–29 months). However, there were 4 non-SCLC (NSCLC) patients with OS longer than 2 years (3 of them are still alive). All the 3 patients received immune checkpoint inhibitor-containing therapy. In 18 patients with SCLC, there were differences in OS among the 3 clusters (p = 0.015) and differences in OS between Cluster II and Cluster I + III (p = 0.034). Similarly, there was a difference among the 3 clusters in 15 NSCLC patients (p = 0.018), and there was a difference in OS between Cluster I and Cluster II + III (p = 0.035).
Discussion
The results of this study are as follows. The frequency of pancreatic metastasis at the time of diagnosis of lung cancer was extremely rare, at 2%. Patients with pancreatic metastases revealed at diagnosis had a median age in the mid-70s. Considering the results that the median age of all lung cancer patients was the mid-60s, pancreatic metastasis was conspicuous in relatively young patients. Of the 33 patients, more than 80% of them were male, and the proportion of females was low. As a result of cluster analysis, patients with were divided into 3 clusters: a cluster characterized by bone metastasis, a cluster characterized by brain metastasis/adrenal metastasis, and a cluster characterized by liver metastasis. This result suggested that pancreatic metastasis in lung cancer patients has specific patterns of metastasis with statistically difference. In this analysis, pancreatic metastases in SCLC patients were strongly associated with bone metastases. Therefore, these patients had a high proportion of patients classified into Cluster I. However, it should be emphasized that this does not indicate that SCLC patients with pancreatic metastasis rarely have concurrent liver metastasis.
The frequency of pancreatic metastasis of lung cancer was more than 10% in post-mortem patients [1]. However, the frequency of pancreatic metastasis from lung cancer diagnosed in ante-mortem was even lower, ranging from 0.59 to 3.3% [4–6]. In a retrospective study by Niu et al. only 17 (0.59%) of 1872 patients of NSCLC showed pancreatic metastases [6]. In our previous study 28 (3.3%) of 850 lung cancer patients had or developed pancreatic metastasis [4]. The difference in the frequencies depended on various conditions such as the presence or absence of pathological diagnosis and the presence or absence of metastasis during the clinical course [4–6].
In our previous study, patients who developed pancreatic metastasis during the clinical course were included in the analysis [4], but this time only the pancreatic metastasis at the time of lung cancer diagnosis was examined because the focus was on examining the ‘pattern of metastasis’. Pancreatic metastases are extremely rare metastases, although they are thought to be highly dependent on the accuracy of diagnostic imaging. In this study, the PET examination performed on some patients was also considered to be an important examination in the future. Surgical biopsy for diagnosis of pancreatic metastasis in patients with multiple metastases is impractical, although pathologic confirmation may be necessary. Cytology of bile juice specimens in an endoscopic approach might be useful. Many patients with pancreatic metastasis had simultaneous metastasis to the bone, brain, and adrenal gland [3, 4]. Although the frequency of synchronous metastatic organs was shown [4], we could not reveal metastatic patterns in our previous study [4]. As for histological types, many reports indicated that the frequency of adenocarcinoma and SCLC was high [4, 27–29], although differences in histology did not affect the treatment of pancreatic metastases. Although extremely rare, there have been reports of pancreatic metastasis in patients with lung squamous cell carcinoma [20, 21, 30], but none of our patients had pancreatic metastasis from squamous cell carcinoma. This might be dependent on the results of the study sample size. In this study, there was no statistical difference in the frequency of pancreatic metastasis between lung adenocarcinoma and squamous cell carcinoma, but a difference in the frequency was found between SCLC and squamous cell carcinoma.
Hepatobiliary stenosis due to pancreatic metastasis makes it difficult to select hepatic metabolic antitumour agents, which might result in limited treatment options. However, with the recent spread of treatments for hepatobiliary tract stenosis, such as stent placement, the number of patients who can receive optimal lung cancer drug treatment is increasing [11, 12]. With these advances in treatment, we once again focused on lung cancer and pancreatic metastasis and performed a cluster analysis of metastasis patterns in patients with pancreatic metastasis. The median OS in our patients was 4 months (range 1–29 months). The prognosis of lung cancer patients remains poor [21, 22, 25], but an increasing number of NSCLC patients might expect longer survival with immune checkpoint inhibitor-containing therapy, as observed in the present study. In addition, although the comparison of background factors other than SCLC and NSCLC differed, the possibility of investigating prognostic differences among clusters of metastases was shown.
There were some limitations in this study. First, the number of patients was small, comprising mainly the data of imaging diagnostics of patients by CT. Only a limited number of patients had pathologic specimens from pancreatic metastatic lesions. In addition, issues of differential diagnosis of patients with synchronous lung and pancreatic cancer or pancreatic disease resembling metastasis also existed. Although there were restrictions as described above, we suppose that our results might contribute to future research. In the present study, we were able to analyse the metastasis pattern in lung cancer patients with pancreatic metastasis for the first time. Clarifying the metastasis pattern is beneficial to patients if it is possible to efficiently carry out imaging tests on sites that are likely to appear in the future and lead to early detection. This study is the first attempt to understand metastasis to other organs as a pattern, and this study is pioneering in this field.
Conclusions
In this study, we showed that specific metastatic organ patterns might exist in lung cancer patients with pancreatic metastasis. Confirmation studies with large numbers of patients are necessary in the future. It is important to collect information on metastatic patterns and utilize it to improve medical care. Because it is supposed that the information is not only of academic interest but also directly related to treatment, it is expected that research in this area will progress in the future.