Introduction
Central hypothyroidism (CH) is a well-known side effect of cancer therapy, generally attributed to cranial irradiation [1, 2]. However, up to 16% of patients with CH have not been previously exposed to radiation therapy [3]. It has therefore been suggested that chemotherapy per se might impair the hypothalamus-pituitary-thyroid axis (HPTA) of childhood cancer survivors [4, 5]. A significant role is attributed to high doses of glucocorticoids that inhibit thyrotropin-releasing hormone (TRH) secretion in the hypothalamus with consequently reduced secretion of thyroid stimulating hormone (TSH) from the pituitary gland or the secretion of an abnormally glycolyzed and therefore inactive form of TSH [6,7].
The symptoms of CH tend to be mild or absent, and diagnostic criteria of CH require an FT4 value below the normal range in addition to a low TSH value [8]. However, some patients with CH have normal TSH values and T4 or FT4 levels within the low part of the normal range. For these reasons, such patients often remain unrecognized. A standard test for identification of CH is abnormal, delayed TSH response in addition to TRH administration [7], an evaluation that in the setting of an ongoing intensive chemotherapy is difficult to perform. Moreover, thyroid hormonal status is not a routine laboratory examination in children undergoing chemotherapy alone and yet another cause for underestimated prevalence of CH among paediatric oncology patients.
Aim of the study
The aim of our survey was to determine the incidence of CH among children with haematological malignancies treated with glucocorticoids, based on clinical (symptoms) and laboratory data (hormonal status). Replacement therapy administration (indication, duration) and resolution of symptoms were also recorded.
Material and methods
A single-centre prospective study was performed of steroid-induced thyroid dysfunction in children and adolescents, aged 0–17 years, treated for haematological malignancies at the Department of Oncology and Haematology in the Children’s Hospital Zagreb, from 1 January to 31 December 2019. The estimated sample size was 25. Epidemiological (sex, age), clinical (diagnosis- acute lymphoblastic leukaemia, Hodgkin and non-Hodgkin lymphoma; symptoms of hypothyroidism- fatigue, apathy, depression, oedema, constipation; hormonal substitutional therapy – levothyroxine, hydrocortisone, fludrocortisone), and laboratory data (serum T3, T4, TSH, cortisol levels) were retrieved from patients’ computer medical records and entered into designated tables. All patients diagnosed with acute lymphoblastic leukaemia (ALL), non-Hodgkin (NHL) and Hodgkin lymphoma (HL), whose parents or guardians signed the informed consent form prior to enrolment were included in the study. Excluded were patients with haematological malignancies, whose standard treatment protocols do not include glucocorticoids (e.g. acute myeloid leukaemia), and those unwilling to participate. The survey was conducted according to the Helsinki Declaration and approved by the institutional review board (Children’s Hospital Zagreb Ethics Committee Approval No. 02-23/9-1-22).
The primary objective of the study was to determine the occurrence of CH among paediatric haematological patients treated with steroids, record the most common signs and symptoms, and document the frequency and duration of hormonal substitutional therapy. The secondary objective was to establish the statistical difference of the aforementioned variables regarding the type and dosage of glucocorticoids. Additionally, concomitant adrenal insufficiency was noted.
Thyroid function status was analysed at the onset (day 1) and cessation, or right before tapering, of steroid therapy (ALL and lymphoblastic lymphoma [LL] – induction (prednisone 60 mg/ m2/d), day 28; re-induction (dexamethasone 10 mg/m2/d), day 21; HL – OEPA (prednisone 60 mg/m2/d) and COPDAC (prednisone 40 mg/m2/d), day 15; NHL – dexamethasone 10 mg/ m2/d, day 5), and randomly at a routine follow-up. Additional diagnostic procedures, including thyroid ultrasound and levels of anti-thyroid autoantibodies, were also recorded, if done.
The diagnosis of overt CH was established when low T4 concentration accompanying inappropriately low or low-normal TSH concentration with symptoms of hypothyroidism was found. Mild forms of CH or possible CH were considered when serum T4 levels were within the low part of the normal range despite a low or normal TSH. No additional investigations were performed to diagnose CH in these patients due to the challenging setting of these patients.
Results
Twenty-one patients, of whom 12 were diagnosed with ALL, 3 with NHL, 2 with LL, and 6 with HL, were included in the study. The study design is presented in Figure 1.
Figure 1
Study design: malignant haematological diagnosis, steroid treatment (type, dosage), overt central hypothyroidism, symptoms, and substitutional therapy presented in a flowchart
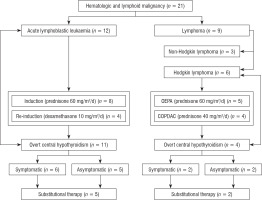
Our subjects were predominately male (61.9%), mean age 9.1 years (5.5 years in patients with ALL, 14 years in patients with lymphoma). Overt CH was determined in 15 patients (71.4%), most of whom were diagnosed with ALL (73.3%). Thyroid hormonal status corresponding to a mild form of CH or possible CH comprised 3 patients (14.2%). Symptoms consistent with hypothyroidism comprised 8 (53.3%) patients with overt CH and one patient with mild or possible CH. The most commonly observed symptoms and signs were fatigue, apathy, and electrolyte imbalance, which were documented in 6 (75%) symptomatic patients. Additional diagnostics (ultrasound, antibodies) was performed in only one patient; functional tests were not carried out.
Overt CH was more prevalent in patients with ALL than in those with HL (χ2 = 7.385, p = 0.025). Among children with ALL, there was no significant difference in the incidence of CH between the dexamethasone and prednisone groups, although dexamethasone-induced CH was more frequently symptomatic (χ2 = 6.000, p = 0.03). The dosage of prednisone (60 mg/m2/d vs. 40 mg/m2/d) in patients with HL had no impact on the incidence of CH or symptomatic presentation.
Concomitant adrenocortical insufficiency was found in 14 (66.6%) patients, with hydrocortisone or fludrocortisone therapy initiated in 13 patients (61.9%). No significant difference was found in the prevalence of adrenocortical insufficiency in patients with ALL regarding the type of steroid therapy, nor in patients with HL regarding the dosage.
Levothyroxine substitution therapy was initiated in 7 (46.6%) children with overt CH and one child with mild or possible CH. The average dose of levothyroxine used was 1.5 µg/kg/per day, while the average duration of levothyroxine therapy was 2.08 ±2.4 months, median 1.25 months. Reported symptoms vanished after a week of substitutional therapy initiation on average. Follow-up thyroid function tests were known in 6 patients treated for hypothyroidism and morning cortisol levels in 11 patients treated for adrenal insufficiency, all showing improved thyroid or adrenal gland function.
Discussion
A wide variety of endocrine complications occur during and after ALL treatment in children, including growth hormone deficiency, adrenal insufficiency, obesity, osteopaenia, and subclinical or clinical thyroid hormone deficiency [9]. A vast majority of the aforementioned disorders have been associated with cranial irradiation [9] and are therefore diagnosed in childhood cancer survivors. Moreover, rarely have acute endocrine disorders such as central hypothyroidism been related to intensive chemotherapy; therefore, thyroid dysfunction as a result of cytostatic administration remains insufficiently recognized [10].
With a prevalence of about 85% in our cohort, CH has been distinguished as a frequent endocrine abnormality during intensive leukaemia and lymphoma treatment, addressing the necessity for the development of a comprehensive diagnostic algorithm. Seldom is CH a result of leukaemia central nervous system (CNS) infiltration [11], but as none of our patients was CNS positive and irradiation was avoided, thyroid dysfunction in all our cases was attributed to glucocorticoid therapy. The specific time-points of hormonal status evaluation in our study were determined based on ample clinical observations and published data on adrenal insufficiency occurrence [12]; namely, the presence of thyroid dysfunction-attributable signs and symptoms correlates to glucocorticoid therapy cessation. However, the optimal diagnostic schedule still needs to be established. Whether thyroid ultrasound and auto-antibody tests should be part of a routine diagnostic approach still needs to be defined, because the results of these exams have often been found normal [4]. Performing hormonal functional tests remains a challenge, not only at the time of extensive initial leukaemia diagnostics, but especially in the setting of many co-existent burdensome therapy-related toxicities and the patient’s deteriorating general condition.
The clinical association of acute leukaemia and autoimmune thyroid disease in adults was established in the early 1990s [13]. Drug-induced CH may also be caused by immunological mechanisms, or in the case of the glucocorticoids, by inhibition of TSH through direct effects of TRH in hypothalamus [7, 14]. Although steroid-induced CH was believed to be clinically insignificant [7], our results show the opposite, with more than half of the patients being symptomatic, mostly complaining of fatigue.
The most common clinical manifestations of hypothyroidism (fatigue, apathy, depression, oedema, constipation) [15] are barely distinguishable from the signs and symptoms of the primary disease or other common treatment side effects in children with haematological malignancies. Because the criteria for initiation and duration of levothyroxine substitution therapy in these patients are still non-existent, we introduced treatment primarily in patients with severe fatigue and apathy, accompanied by both low levels of T4 and TSH, which were therefore believed to be aggravated by thyroid dysfunction. In addition, refractory hyponatraemia, resistant to sodium replacement and fludrocortisone, was another reason for levothyroxine substitution, because hypothyroidism is listed as one of the possible aetiologies of hyponatraemia [16]. Low serum sodium levels have been described in moderately and severely hypothyroid patients [17], but other possible causes of hyponatraemia should be ruled out before the causative link is established [18]. However, in our experience, when other conditions (i.e. salt-wasting nephropathy) have been excluded and no improvement in restitution of serum sodium levels has been achieved with fludrocortisone therapy in the setting of concomitant adrenal insufficiency, good clinical and laboratory response has been observed only when levothyroxine was administered. The duration of levothyroxine replacement therapy varied but, once introduced, it lasted until normalization of T4 and TSH levels.
The significantly higher occurrence of symptomatic presentation of CH in the dexamethasone group was not surprising, because prednisone is associated with fewer adverse events in the treatment of ALL compared to dexamethasone [19]. CH being more frequent in the ALL group than in the HL group, on the other hand, might be attributable to longer, multiple-week glucocorticoid use in current ALL treatment protocols.
Adrenal insufficiency affects almost all children with ALL upon cessation of glucocorticoid therapy. Randomized controlled trials have determined no difference in the frequency of adrenal insufficiency in children with ALL regarding the type of glucocorticoid therapy [12]. Supraphysiological glucocorticoid doses suppress the hypothalamus-pituitary axis, causing an impaired stress response, which may be life-threatening in circumstances such as fasting or infections, thus indicating the benefit of hydrocortisone replacement [12].
We are aware of the possible limitations of our study. First, additional laboratory (anti-thyroid autoantibodies, functional test results) and imaging (ultrasound, MRI of the brain) findings were not provided. Also, in a larger cohort prospective study scheduled at our department, free T4 and free T3 in addition to TSH will preferably be used. Second, the time-points of laboratory re-evaluation, especially in the follow-up, might not have been ideally selected. Third, due to the lack of guidelines, the decision on replacement therapy administration was made by the attending physician based on the general institutional consensus of introducing therapy to moderately to severely affected patients, but it was influenced by personal opinion and professional beliefs. Moreover, a small-scale sample size compromises derived conclusions. However, we believe the results of our study will provoke discussions on diagnostics and management of acute endocrine complications and consequently improve supportive care in children with haematological malignancies.
Conclusions
Further studies are needed to determine the real incidence of thyroid dysfunction during intensive chemotherapy treatment in children with ALL and lymphoma. A comprehensive diagnostic approach needs to be established soon. Recommendations for optimal hormonal replacement therapy and a follow-up plan for paediatric oncology patients with CH are also urgently required. Hopefully, an ongoing, larger, and structured prospective survey in our centre, among others, will provide answers to some of the unsolved issues.

 POLSKI
POLSKI






