Introduction
Type 1 diabetes (T1D) is a worldwide epidemic that has led to a rise in diabetic nephropathy (DN). The main biochemical disorder in diabetes and DN is hyperglycaemia, causing a toxic effect via activation of the chain of different intracellular enzymatic reactions, inflammation, and oxidative stress [1].
Diabetic nephropathy, also known as diabetic kidney disease, is the chronic loss of kidney function occurring in patients with diabetes mellitus. Diagnosis of DN is based on the measurement of abnormal levels of urinary albumin in diabetic patients after the exclusion of other causes of albuminuria. Two out of 3 samples falling within the microalbuminuria (30 to 300 mg of albumin/24 h) or macroalbuminuria (more than 300 mg of albumin/24 h) range confirm the presence of DN [2].
Adequate control of blood glucose in patients with diabetes is a critical aspect of their therapy. Glucose present in the blood in excessive concentrations can enzymatically bind to proteins such as haemoglobin and causes glycosylation. The level of glycosylated haemoglobin formation depends on the concentration of glucose in the surrounding environment. People with high blood glucose levels will have higher levels of glycated haemoglobin. Non-enzymatic glycation that occurs as a result of spontaneous interaction between glucose and amino groups of proteins leads to the formation of advanced glycation products (AGA). AGA in parallel with other metabolic disorders causes functional and morphological kidney damage in diabetic kidneys [3, 4].
The role of endothelin-1 (ET-1) in the kidney has been under study for many years. However, the complex mechanisms by which endothelin controls the physiology/pathophysiology of this organ are not fully resolved. Podocytes produce, secrete, and bind ET-1. Podocytes are critical determinants of the integrity of the glomerular filtration barrier, and their injury leads to proteinuria, glomerulosclerosis, and progression of renal disease [5]. Endothelial cells are considered a principal source of ET-1 within the glomeruli [6].
However, the importance of endothelium-podocyte crosstalk as a major determinant of a healthy filtration barrier and hence the cellular location of ET-1 production and function in T1D and DN, as well as its general effect on the cardiovascular system, is not clear. Autocrine and/or paracrine ET-1 signalling is considered to be crucial in contributing towards the development of albuminuria.
ET-1 has also been shown to play a role in numerous pathophysiological mechanisms within the glomeruli. Its role is shown in the pathogenesis of many forms of chronic kidney disease such as diabetic nephropathy [7], sickle nephropathy [8], and focal segmental glomerulosclerosis [6]. To address the topic of whether ET-1 levels changed in patients with early stages of T1D and DN we measured ET-1 levels in T1D patients and patients with DN.
It is noteworthy that, especially in adolescents, microvascular complication frequency is more than expected 2–5 years after initiation of T1DM [4]. In the latest global consensus report published in 2018 by the International Society for Paediatric and Adolescent Diabetes (ISPAD) and International Diabetes Foundation (IDF), it is recommended that annual screening for microvascular complications, including albuminuria, glomerular filtration changes should be started from age 11 years and after 2 years of diabetes duration.
Appropriate screening for complications in diabetic patients, glycaemia levels, and blood pressure control are key in T1D management [9, 10]. In the present study we analysed levels of GFR, ET-1, cholesterol, and lipid oxidation ratio, and their dependence on the main clinical parameters (disease course, blood pressure, HbA1c, albuminuria) in children with T1D and DN. In order to evaluate the most initial pathological changes related to metabolic and functional disorders we included analysis of patients with T1D disease duration ≤1 year. The aim of the study was to evaluate the most initial metabolic and functional disorders in diabetic children and children with DN.
Material and methods
A survey of 76 children (33 males, 43 females) with T1D and DN aged 3 to 17 years in the Endocrinology Unit of Clinical Paediatric Hospital No. 6 (Kyiv, Ukraine) was performed. Informed consent was obtained from all patients and their families. The study was approved by the hospital’s local ethics committee. All informed consents were signed by the children (≥12 years old) themselves and/or by their parents and kept in their medical records. The study was conducted in Clinical Paediatric Hospital No. 6 where the Clinical Base of the Department of Paediatrics No. 4 of Bogomolets National Medical University is located. The control group included 27 healthy children (12 males and 15 females). Patients’ inclusion and exclusion criteria are given in Table I.
Table I
Patients’ inclusion and exclusion criteria
Complex examination including conventional methods (physical examination, blood pressure measurement, blood tests, study of urinary sediment, renal ultrasound, ECG etc.) was done for all patients.
Urinary albumin excretion measured in 24-hour urine collection samples used the basic conventional technique established in Clinical Paediatric Hospital 6. Endothelin-1 levels were measured using ELISA assay and commercially available Endothelin-1 ELISA kit (Abcam, USA). Lipid oxidation ratio recorded using a spectrophotometer T70 UV/VIS (PG Instruments Limited, UK). Quantitative evaluation of spectra was performed using the software Image J (NIH, USA).
The results were processed using the methods of variation statistics (STATISTICA 6.0) and non-parametric statistical approaches (Mann-Whitney test). Spearman’s correlation coefficient was used to express associations between parameters. The results are presented as mean ± SEM and were considered statistically significant the level of p < 0.05. Data that were qualified to enter the multivariate analysis were analysed in multivariate logistic analysis. Statistical analysis was performed using the statistical software package SPSS version 20 for Windows. P values < 0.05 were considered to be statistically significant.
Results
The study was designed as an analysis covering data from 2014 to 2020 including children with T1DM followed in the Endocrinology unit of Clinical Paediatric Hospital No. 6 (Kyiv, Ukraine). The T1D group comprised patients with an average disease duration of 9 ±2.13 months. The group of children with DN included patients with disease duration of at least 1 year. The average disease duration in this group was 6.2 years.
All patients were seen every 3 months, and all were on multiple flexible dosing intervals of insulin treatment. Chronological age, diabetes duration, weight, height, body mass index (BMI), blood pressure, HbA1c, and serum cholesterol were recorded during each visit to hospital. WHO BMI-for-age z-scores for boys and girls were used to assess the BMI values in children included in the study. Children were considered to have a normal body weight once their weight-for-height Z-scores were observed within the interval 0 to < ±1. Based on basic clinical data, all patients were divided into groups (Table II).
Table II
Clinical characteristics of patients
The glomerular filtration rate (GFR) was used to assess kidney function. The Schwartz formula for children and adolescents 1 to 17 years old was used [10]:
eGFR = 0.413 x (height/Scr) if height is expressed in centimetres
OR 41.3 x (height/Scr) if height is expressed in metres
eGFR (estimated glomerular filtration rate) = ml/min/1.73 m2
Scr (standardized serum creatinine) = mg/dl
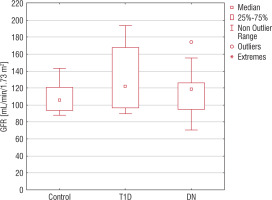
Our results show that in the control group the GFR value was 103.94 ±4.95% ml/min/1.73 m2. In contrast, in the T1D group the GFR value was 129.78 ±5.64% ml/min/1.73 m2, which is statistically different compared to control group value (p < 0.01). The GFR level in the DN group was higher compared to the control group value (113.74 ±3.52 ml/min/1.73 m2 vs. 103.94 ±4.95% ml/min/1.73 m2, p < 0.01) (Figure 1).
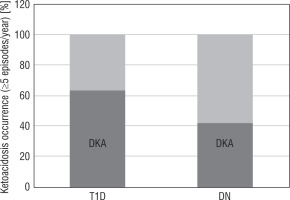
Interestingly, in the T1D group 63.2% of patients had 5 and more episodes of decompensation, i.e. diabetic ketoacidosis (DKA) per year causing hospitalization. In contrast, 41.9% of patients from the DN group were diagnosed with 5 and more episodes of KDA per year (Figure 2).
Blood pressure changes in children with T1D as well as in the group with DN were evaluated.
Systolic blood pressure was significantly lower in children with T1D as compared to the control group – 97.14 ±3.8 mm Hg and 111.34 ±0.88 mm Hg, respectively (p < 0.01). The DN group value was significantly higher than in the control group and in patients with T1D (p < 0.01). Diastolic blood pressure values were similar in the control group and in the group of patients with T1D, at 64.15 ±1.5 mm Hg and 64.89 ±1.45 mm Hg, respectively. However, the DN group value is higher than in the control group and in the group of patients with T1D (p < 0.01).
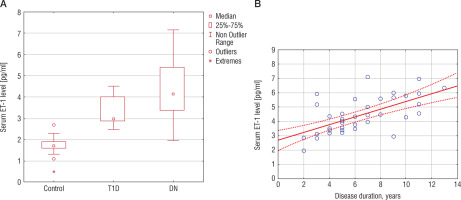
In order to evaluate the possible effector in the development of microvascular disorders and BP changes we measured the level of ET-1. All patients with T1D included in the study had homogenous results of ET-1 levels. Differences within the group or their dependence on disease duration were not evaluated. In patients with T1D the level of ET-1 was significantly higher compared to the control group value – 1.71 ±0.1 pg/ml and 3.41 ±0.12 pg/ml, respectively (p < 0.01). Patients from the DN group had higher levels of ET-1 (4.38 ±0.2 pg/ml) compared to the T1D group (p < 0.001) (Fig. 3A). We found a positive direct relationship between ET-1 levels and disease course in children with DN (r = 0.65, p < 0.05) (Fig. 3B).
Figure 3
Serum ET-1 in children with TD1 and DN (A). Correlation between serum ET-1 and disease duration in patients with DN
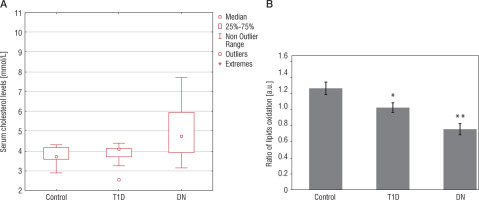
We measured levels of blood cholesterol in all patients included in the study. Our results show that the control group cholesterol value was 3.81 ±0.07 mmol/l, and in the T1D group – 3.86 ±0.08 mmol/l. In contrast, the DN group value was 5.11 ±0.27 mmol/l, which is higher in comparison to the control group and T1D group values (p < 0.01) (Fig. 4A).
Furthermore, we examined children for plasma level of the lipid oxidation ratio. The ratio shows the fractions of non-oxidized/oxidized lipids. In the control group the value is 1.27 ±0.08. In the group of children with T1D – 1.03 ±0.06%, which is lower as compared to the control group value (p < 0.05). In children with DN the lipids oxidation level was 0.76 ±0.07%, which is lower as compared to the control group value (p < 0.01) (Fig. 4B).
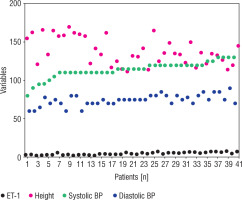
In the DN group the values of systolic and diastolic blood pressure were higher as compared to T1D groups values. At the same time, the average value of height was somewhat higher in patients with DN in comparison to the T1D group values. An association between systolic BP, diastolic BP, height, and serum ET-1 in patients with DN was found. The serum ET-1 level was independently associated with increased values of systolic BP (4.81 ±1.43, 95%: 0.64 to 2.67, p < 0.05) and diastolic BP (1.81 ±1.03, 95%: 1.69 to 5.15, p < 0.04) in children with DN. Height values did not show a positive association with increased values of systolic BP (–0.02 ±0.006, 95%: –0.11 to 0.11, p = 0.84) and diastolic BP (–0.09 ±0.07, 95%: –0.23 to 0.04, p = 0.82) in children with DN (Table III). Figure 5 shows the individual distribution between the above-mentioned parameters in children with DN.
Table III
Multivariate logistic regression model evaluating the association between systolic BP, diastolic BP, height, and serum ET-1 in patients with DN
Discussion
The Diabetic nephropathy is a life-threatening complication of type 1 diabetes [11, 12]. A quarter of T1D patients with DN progress to ESRD [3]. DN is also a major risk factor of coronary artery disease (CAD) development [13] and overall mortality in diabetic patients. The natural history of DN is characterized by a long silent period without any prominent clinical signs and symptoms of the disease. This leads to a delay of the treatment and development of early complications. It was reported that only a third of patients aged < 20 years with DN were administered ACE inhibitors (ACEi) and/or angiotensin-receptor blockers (ARB), which is supposed to be a basic renoprotective treatment [14].
Late start of the DN treatment leads to irreversible kidney damage – GFR decline and renal structural changes. These, in turn, are factors leading to ineffectiveness of therapeutic strategies [15]. Earlier identification of GFR decline is an important issue, which allows the therapeutic interventions to decrease the rate of GFR loss and prolong the time to development of end-stage renal disease (ESRD).
There are data showing the benefit of the early and adequate glycaemic and blood pressure control in preventing microvascular complications in T1D [16, 17]. Vascular disorders, including hypertension, is one of the causes of death in patients with ESRD in diabetic patients [17, 18]. However, it is not clear whether hypertension can cause gradual loss of kidney function. Moreover, it is unclear how these disorders are inter-related in their development and progression.
Vupputuri et al. found a direct association between high BP and GFR decline. The age-related decline in GFR is the most important predisposing factor of CKD [19]. There are data showing an association between baseline blood pressure (BP) and subsequent GFR decline and/or development of CKD. However, some authors shown no relationship between these factors [20].
In our study we found that children with T1D and disease duration < 1 year have a 25% increase of GFR as compared to control group values. At the same time, these patients have normal BP values and increased serum ET-1 levels. In our previous studies [21, 22] we found that patients with T1D have high levels of intracellular hypoxia (HIF-1alfa, marker) and apoptosis effectors. We hypothesise that increased GFR might be a response to metabolic changes that have a case in T1D, i.e. hyperglycaemia, hypoxia, apoptosis, and ET-1 activation. Moreover, we analysed a number of decompensation episodes (DKA) in these patients and found that the T1D group had high numbers of DKA (≥ 5 episodes/year), which may be one of the reasons for the metabolic effect on kidney and “shock” GFR increase.
Patients with signs of DN show increased levels of GFR. However, the GFR value in this group was lower as compared to T1D patients. In children from the DN group GFR increased by 12% as compared to the control group value. All children with DN have increased systolic BP values, higher level of serum ET-1 (as compared to the T1D group), and increased cholesterol values. Taking into account all these changes, we hypothesize that arteries and veins become damaged later and this effect depends on disease duration. The proof of later is the direct positive relationship between serum ET-1 level and disease course in children with DN. Multivariate analysis shows an association between systolic and diastolic BP values and serum ET-1 levels in children with DN.
In contrast to the well-known role of plasma ET-1 as a contributor to renal and vascular damage, ET-1 is generally believed to act in an autocrine rather than in an endocrine fashion. It is known that circulating ET-1 is filtered by the glomerulus and then destroyed by neutral endopeptidases in the proximal tubule [23]. Thus, we speculate that ET-1 has a direct effect on kidney vasculature in children with T1D and DN via its autocrine effect. The endocrine fashion of the ET-1 activity leads to blood pressure changes, and this effect depends on disease duration.
The American Diabetes Association (ADA) recommends screening for signs of DN in 5 after the onset of T1D. Taking into account the fact that DN is linked to hypertension, patients’ blood pressure should be monitored carefully. In the current study we followed the rule of yearly monitoring of BP, GFR, HbA1c, and ET-1. We believe that this is reasonable in terms of yearly functional and metabolic disorder evaluation and further adequate treatment administration.
In was shown that in paediatric patients with T1D an end-stage renal disease occurred in 2.9% of its population. This was directly associated with poor glucose control (A1C ≥ 10%), higher LDL cholesterol (>100 mg/dl), and age > 6 years at diagnosis [24]. In our study the average disease course in children with DN was 6.2 years. Higher cholesterol levels as well as higher lipid oxidation ration was detected within 5–6 years of the disease duration.
It is known that in youths with type 1 diabetes the structural damages observed in the glomeruli, interstitium, and vasculature present long before the prominent sign of DN. Moreover, functional changes often reflect advanced disease [25]. The Oxford Regional Prospective Study reported that microalbuminuria is associated with poor glycaemic control and higher GFR at 5 years [28]. Albuminuria is a commonly used marker to detect the kidney disease; however, this may be an imperfect marker [26, 27]. Thus, it is advisable to use complex examination in terms of early signs of metabolic functional changes in T1D and DN.
Potential limitations of this study include insufficient evaluation of specific markers of kidney injury – kidney injury molecule (KIM-1), gelatinase-associated lipocalin (NGAL) – in patients with DN and during its “silent period”. The study did not take into account other factors that potentially have an effect of DN formation – hormonal status, gender, and inflammatory markers. Another disadvantage of this study involves the limited number of patients included in the study.
Taking into account our results, we conclude that initial functional changes, i.e. GFR increase, in children with T1D appear during the first year of disease. These early disorders appear ahead of BP changes and albuminuria. The latter is a sign of DN and leads to further albumin-induced damage of the kidney. We hypothesis that early functional disorders in children with T1D are due to metabolic changes that take place in T1D, i.e. hyperglycaemia, hypoxia, and decompensation episodes. DN in children with T1D is associated with the above-mentioned metabolic disorders, ET-1 levels increase and lipid disorders, which we speculate have an effect kidney vasculature and blood pressure. These effects depend on disease duration.
Finally, we focus our attention on fact that DN is characterized by a long silent period of the disease course without signs or symptoms. There is an urgent need for improved methods of detecting early mediators of renal injury, to further prevent initiation and progression of DN. We made a great deal of effort studying these issues in children with T1D and DN. In particular, we speculate that early complex evaluation and monitoring of the above-mentioned changes may help to understand the deep initial mechanisms of T1D progression and its complications development.
Potential limitations of this study include insufficient evaluation of specific markers of kidney injury – kidney injury molecule (KIM-1) and gelatinase-associated lipocalin (NGAL) – in patients with DN and during its “silent period”. The study did not take into account other factors that potentially have an effect of DN formation – hormonal status, gender, and inflammatory markers. Another disadvantage of this study involves the limited number of patients included.

 POLSKI
POLSKI