Introduction
The presence of accessory breast tissue is recorded in about 2–6% of the general female population [1, 2]. In the vast majority of patients, this is an asymptomatic mass located most often in the axillary fossa, resembling an enlarged axillary lymph node. Other, less frequent locations of ectopic breast tissue include the infraclavicular region, the vulva, and the epigastrium [2]. The history of women presenting with accessory breast tissue often includes complaints of an axillary mass associated with discomfort and local erythema that frequently follows the physiological changes of normal breast tissue during the menstrual cycle and pregnancy. Carcinoma of the accessory breast tissue (CABT) is an extremely rare occurrence, representing 0.3% of all breast malignancies [3]. Herein, we present the case and treatment of a 65-year-old woman with a palpable axillary mass that was later diagnosed as ectopic breast carcinoma.
Case report
A 65-year-old, postmenopausal woman was referred to our Breast Clinic complaining of a palpable, growing, and painful mass in her right axilla. Physical examination revealed a palpable tender mass, approximately 3 ×3 cm in size, with visible retraction of the overlying skin area. Physical examination of the breast revealed no palpable lesions. The rest of the physical examination was otherwise unremarkable. The patient’s history revealed a history of primary hypertension, dyslipidemia, gastric ulceration, and major depressive disorder. No family history of breast cancer was reported by the patient. Core biopsy of the mass was promptly scheduled, and the histological report came back positive for Nottingham Grade II NST invasive carcinoma of the breast. Immunochemical staining showed positivity for ER and PR receptors, a negative c-Erb-B2 score, and a Ki67 index of 15%, which were indicative of a Luminal A subtype.
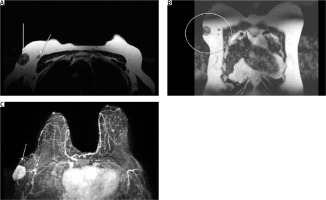
Evaluation of the axillary mass was done utilizing breast magnetic resonance imaging (MRI) for local staging (Fig. 1). Magnetic resonance imaging revealed a 3 × 2.4 × 3.3 cm superficial mass, which showed signs of malignancy, and infiltration of the axillary skin located anterior to the subscapularis muscle, and lateral to the major and minor pectoralis muscles. As part of the staging process, computed tomography (CT) scans of the head, chest, and abdomen were ordered, as well as bone scintigraphy, revealing no additional metastatic lesions.
Fig. 1
(A) Axial and (B) coronal views of the lesion and a suspicious node. Axial STIR view of the lesion
The multidisciplinary team that assessed our patient was composed of breast surgeons, radiologists, oncology specialists, and nuclear medicine specialists. At the time of diagnosis, our institute could not offer immediate oncological consultation due to current public health measures. Our patient was therefore referred to a radiation oncology specialist in a different center, who was however included within the decision-making process. All of the specialists agreed that the best course of action would be for the patient to be scheduled for oncoplastic surgery. The patient underwent breast-conserving surgery and concomitant axillary lymph node dissection (ALND) for removal of the malignant mass (Fig. 2). Axillary lymph node dissection was elected as the best surgical approach for the axilla, due to the proximity of the malignancy to the axillary nodes, in addition to the presence of a suspicious node in preoperative imaging. Both these factors contributed to the decision to surgically treat for advanced axillary disease. Care was taken to preserve the axillary vein and the long thoracic nerve. Closure of the axillary incision required mobilization of skin flaps to ensure optimal cosmetic results.
Fig. 2
(A, B) The final retrieved specimen, containing the carcinoma and (C) the axillary anatomical space post-axillary lymph node dissection
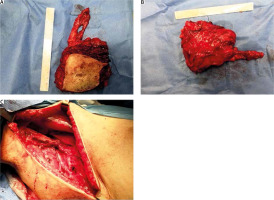
The final pathology report of the removed specimen confirmed the removal of the malignant tumor, along with 3 additional surrounding carcinomatous foci. Despite preoperative MRI imaging reports that commented on possible skin infiltration, the carcinoma was found to extend marginally below the dermis. In total, nine lymph nodes were removed, two of which were infiltrated, one with extra-nodal spread. The final pathological staging was pT2N1a. The postoperative course of the patient was uneventful and she was discharged with a referral to the oncology department to commence adjuvant chemotherapy treatment. A PET-CT scan 3 months postoperatively revealed no additional signs of disease.
Discussion
In total, less than 200 reports of carcinoma in the ectopic breast tissue have been described in the literature. The scarcity of such cases, as well as the unavailability of large case series, accounts for the lack of specific guidelines regarding this rare occurrence. Presentation of the lesion is mostly as a firm mass, which must be distinguished from nodal metastasis, reactive lymphadenopathy, hidradenitis, and lipomas [2, 4]. Published reports on CABT state that mammographic imaging is of limited use in the diagnosis of malignancy due to the localization of the axillary breast tissue away from the field of view [5–7]. Despite its localization, however, mammographic evidence of malignancy in aberrant breast tissue has been previously reported as the first indication of CABT. In a series of 10 reported cases, 6 patients reported mammographic findings consistent with carcinomas and were successfully diagnosed with CABT [8]. Traditional mammography is able to detect enlarged axillary lymph nodes in some cases, but axillary nodal disease is best evaluated by ultrasound [7, 9]. Authors have also reported that oblique and craniocaudal mammographic views can be useful in ectopic breast depiction [10, 11].
In the past, breast surgeons reported total mastectomy as the procedure of choice. However, more recent reports all seem to agree that there is no evidence in favor of this approach unless otherwise indicated, since wide excision of the ectopic gland did not demonstrate worse prognosis [2, 3, 12, 13].
On the other hand, ALND is almost always incorporated within the treatment of breast cancer of the ectopic tissue regardless of the nodal status of the patients [4, 14–16]. The rationale behind this relates mainly to the proximity of the tumors to the axillary lymph nodes, which makes invasion and metastasis much more likely than in breast cancer. Indeed, there is circumstantial evidence of earlier spread to axillary lymph nodes [12, 15, 17]. Again, it must be noted that this is an informally preferred approach, and no data comparing ALND to simple sentinel lymph node biopsy in breast cancer of accessory breast tissue yet exist. The prognosis of CABT is another area of debate. Authors have suggested that the proximity to axillary lymph nodes and the evidence of earlier nodal metastases indicate that CABT patients fare worse than breast cancer patients [14, 15, 17].
Conclusions
Despite the ectopic breast tissue being a largely benign and infrequent occurrence, the breast surgeon must remain vigilant for the possibility of CABT development. At any rate, further epidemiological studies incorporating as many patients as possible are required to construct recommendations on the management and prognosis of CABT. Until such guidelines exist, excision of the carcinoma, along with ALND performance, is a reasonable and justified approach to the surgical treatment of CBT.










