Summary
In the current study, we evaluated a total of 222 ST-elevation myocardial infarction (STEMI) patients, 74 COVID-positive (group I) and 148 COVID-negative (group II), for a mean follow-up of 6 months, and we identified the implications of COVID-19 pandemic on six-month mortality and clinical outcomes. This study differs from previous studies in that it included all consecutive STEMI patients who presented to our hospital, and we extended the follow-up beyond the in-hospital outcomes to a longer period. We also applied propensity score matching to yield two groups in a 1 : 2 ratio to strengthen the study’s results. Additionally, we identified the predictors of 6-month mortality in STEMI patients during the COVID-19 pandemic, and we shed light on the increased incidence of thrombotic complications in the COVID positive patients that may lead to a change in the management strategy of those high-risk groups of patients.
Introduction
The evolution of the coronavirus (SARS-CoV2) pandemic in December 2019 represented a worldwide healthcare challenge. Although absence of symptoms or mild symptoms are the most common presentations in COVID-19 patients, more hazardous symptoms, including pneumonia and acute respiratory distress syndrome, were reported in a significant number of patients [1].
Different mechanisms have been postulated to explain the cardiac symptoms caused by COVID-19 infection, including direct myocardial injury triggered by hemodynamic instability, hypoxia, myocarditis, and thrombosis; as well as multisystem inflammatory syndrome (MIS), which leads to cytokine storm, plaque instability, and plaque rupture [2, 3].
ST-segment elevation myocardial infarction (STEMI) may represent an extreme form of myocardial injury detected by electrocardiography [4]. Combining COVID-19 infection with STEMI represents a challenge as the management strategy differed from how we were used to diagnosing and treating these conditions [5].
Some advocated relying on pharmacological reperfusion at the beginning of the pandemic in order to limit the delay in reperfusion and provide protection for healthcare workers [6]. However, increased rates of unsuccessful reperfusion with the resultant increased rates of mortality and heart failure were the major drawback of this strategy [5, 7].
Studies of those risky patients primarily focused on in-hospital clinical outcomes and in-hospital mortality, but how these patients progress after their discharge was outside the scope of many studies. Additionally, most studies were done when the management strategies represented a controversy between different cardiological societies.
Aim
Our study aimed to provide real-time clinical management and in-hospital outcomes for STEMI patients treated at Benha University Hospital. We also offered 6 months follow-up of patients looking for predictors of poor clinical outcomes after hospital discharge in patients admitted for STEMI combined with COVID-19 infection.
Material and methods
Study population
This prospective observational study was conducted on consecutive STEMI patients who presented to Benha University Hospital (Qalyubia, Egypt) in the period from April 1, 2021, to October 31, 2021. The study was approved by the local Benha University Ethical Committee and informed written consent was obtained from all patients who participated in the study.
We initially included a total of 654 acute STEMI patients presenting within 12 h of the onset of symptoms. STEMI was diagnosed on the basis of typical anginal pain or anginal equivalent symptoms lasting more than 20 min with electrocardiographic (ECG) changes (ST-segment elevations of ≥ 1 mm in ≥ 2 contiguous leads other than V2-3 (where the following cut-points apply: > 2 mm in men > 40 years, > 2.5 mm in men < 40 years, or > 1.5 mm in women regardless of age) or new onset left bundle branch block) with a rise in cardiac biomarkers [8]. Forty-eight patients were excluded from the study by the following exclusion criteria: (1) patients who had undergone prior coronary artery bypass graft (CABG) surgery, (2) patients with a history of ischemic cardiomyopathy with severely impaired left ventricular ejection fraction (LVEF) < 20%, (3) patients who received conservative medical therapy, (4) patients who refused to participate in the study, and (5) patients lost to follow-up.
The enrolled 606 patients were divided into COVID-19 and non-COVID-19 STEMI groups (group I and group II, respectively). We applied propensity score (PS) matching to identify a cohort of non-COVID-19 patients with comparable baseline clinical characteristics to COVID-19 (1 : 2 ratio of COVID to non-COVID patients). This yielded a total of 222 patients (74 in group I and 148 in group II) who were included in the final study analysis.
Baseline and in-hospital evaluation
Baseline data were collected from the patients and their relatives, including age, gender, coronary risk factors (hypertension, diabetes, dyslipidemia, smoking, body mass index (BMI), history of ischemic heart disease (IHD), or prior stroke), time from symptom onset to presentation, associated co-morbidities, baseline physical examination, admission Killip class, site of infarction as determined by ECG, and the laboratory data.
The in-hospital management of the study population was also evaluated, including medications used during hospitalization, reperfusion method, comprehensive echocardiographic examination, total length of hospital stay, TIMI risk score, and in-hospital clinical outcomes.
Primary percutaneous coronary intervention (PCI) through femoral access was the preferred reperfusion strategy whenever feasible. However, during the COVID pandemic, primary PCI was not readily available at all times, and we used thrombolytic therapy to achieve early patency of the infarction-related artery (IRA) with planned rescue PCI in case of failed fibrinolysis.
Streptokinase was the fibrinolytic agent used during the study and was combined with low molecular weight enoxaparin. In patients treated with primary PCI, clopidogrel was given in a 600-mg loading dose followed by 150 mg daily for 1 week, then 75 mg daily thereafter. During the PCI procedure, patients received unfractionated heparin (100 IU/kg), with a reduction of the dose to 70 IU/kg in the case of administration of a glycoprotein IIb/IIIa inhibitor (eptifibatide). An aspirin loading dose of 300 mg followed by 75–100 mg/day was given to all patients. A 12-lead ECG was obtained 45–90 min after fibrinolytic therapy or immediately after the PCI procedure and was compared to the admission ECG [9]. The arithmetic sum of ST-segment elevation measured at the J point was calculated. ST-segment resolution (STR) was classified as complete STR (regression ≥ 70%) or incomplete STR (unaltered or worsened ST elevation or regression < 70%) [10]. Total ischemic time was identified as the time from the onset of persistent symptoms to the time of reperfusion.
A conventional echocardiographic examination was performed as soon as the patient was stabilized by an operator blinded to the study groups. The LV diameters and wall thicknesses were measured in the left parasternal long axis at the level of the mitral valve tips, ensuring a measurement perpendicular to the long axis of the ventricle. End-diastolic (EDV) and end-systolic volumes (ESV) were used to calculate left ventricular ejection fraction (LVEF) using the modified biplane Simpson’s method in the apical four- and two-chamber views as recommended by the American Society of Echocardiography [11]. The 17-segment model of the left ventricle was used. Myocardial segments were graded according to their wall motion as normokinetic (grade 1), hypokinetic (grade 2), akinetic (grade 3), or dyskinetic (grade 4), and the wall motion score index (WMSI) was calculated [12]. If present, the severity of mitral regurgitation (MR) and tricuspid annular plane excursion (TAPSE) were assessed according to the guidelines [11, 13].
Clinical end points and definitions
COVID-19 patients were identified when a PCR assay for SARS-CoV-2 was positive during or within 1 month before the index STEMI hospitalization. Primary PCI was defined as PCI within 12 h of symptom onset in a patient not receiving fibrinolysis [14]. Rescue PCI was defined as PCI mandated by persisting symptoms or persisting ST-segment elevation (failure to achieve ≥ 50% ST resolution) within 90 min after the administration of fibrinolysis [14].
The study’s primary endpoint was the occurrence of major adverse cardiac events (MACE; composite of death from any cause, recurrent MI, target-vessel revascularization, and stroke) at 6 months. The secondary endpoints included death from any cause, recurrent MI, target-vessel revascularization, stroke, major bleeding, and thrombotic complications.
Recurrent MI was defined as repeated clinical symptoms or development of new ECG changes associated with a new rise of cardiac troponin (cTn) values > 99th percentile upper reference limit (URL) in patients with normal baseline values or an increase of cTn values > 20% of the baseline value when it is above the 99th percentile URL [8]. Ischemia-driven target vessel revascularization (TVR) is repeated revascularization with PCI or CABG of the IRA driven by symptoms or objective evidence of ischemia [9]. Thrombotic complications included the composite of myocardial infarction, acute ischemic stroke, limb ischemia, mesenteric ischemia, pulmonary embolism, and deep vein thrombosis. Major bleeding was defined as type 3 or 5, according to the Bleeding Academic Research Consortium [15].
Statistical analysis
Statistical data were presented as means and standard deviations for continuous variables. Categorical variables were presented as numbers and percentages. The independent t-test or the Mann-Whitney U-test was used for continuous variables to compare the groups. The categorical variables were compared using the exact χ2 test or Fisher’s exact test as necessary. Propensity score (PS) matching was applied to identify a cohort of COVID-19 patients with comparable baseline clinical characteristics to non-COVID-19 (1 : 2 ratio of COVID to non-COVID patients). A propensity score was first estimated for each patient using a multiple logistic regression model that included the 12 covariates shown in Table I. The matching was then performed using a greedy matching protocol (1 : 2 nearest neighbor matching without replacement) with a caliper width of 0.2 of the SD. Analyses in the PS-matched cohort were then performed using a paired t-test, or the Wilcoxon signed rank test for normally and non-normally distributed continuous variables, respectively; and categorical variables were compared using McNemar’s or Bowker’s test of symmetry, as appropriate.
Table I
Baseline characteristics of study cohort
A multivariable logistic regression model was used to evaluate the independent predictors of 6-month mortality in STEMI patients during the COVID-19 pandemic. Variables that displayed a marginal association on univariable testing (p ≤ 0.20) were entered into the regression model. The variables entered in the regression model were: COVID-19 infection, age, gender, coronary risk factors (hypertension, diabetes, BMI, dyslipidemia, smoking, history of IHD, or prior stroke), presenting features (heart rate, systolic and diastolic blood pressures, Killip class > 1, and main presenting symptoms), total ischemic time, site of infarction, reperfusion strategy, laboratory findings (serum creatinine, C-reactive protein, and high-sensitive troponin T levels), echocardiographic parameters (LVEF%, WMSI, severe MR, and TAPSE), ST segment resolution, TIMI risk score, and occurrence of arrhythmias (atrial fibrillation, bradyarrhythmias, and malignant arrhythmias).
Only variables of significant correlation with mortality (p < 0.05) were included in the final regression model. In order to assess the predictive power of each variable, the odds ratio (OR) and its 95% confidence intervals (CI) were measured. All the p-values were two-sided. P-values of < 0.05 were regarded as statistically significant. Data management and statistical analysis were carried out using SPSS vs. 21 (IBM, Armonk, New York, the United States).
Results
Baseline clinical characteristics
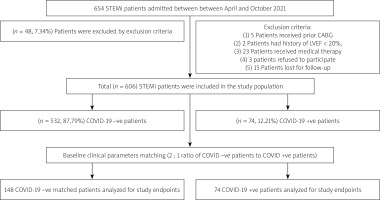
As shown in Figure 1, a total of 606 STEMI patients were initially enrolled in this study. After performing PS matching, 74 COVID-19-positive (COVID +ve) STEMI patients were included in group I and compared with 148 COVID-19-negative (COVID –ve) STEMI patients (group II).
Figure 1
Flowchart of the present study population
STEMI – ST-elevation myocardial infarction, CABG – coronary artery bypass graft, LVEF – left ventricular ejection fraction.
There were no significant differences in the baseline-matched clinical characteristics between the two groups (Table I). However, COVID +ve patients presented more often with equivalent angina symptoms, higher admission heart rate, lower diastolic blood pressure, and higher Killip class. Additionally, higher levels of high-sensitive cardiac troponin T and serum C-reactive protein were significantly associated with COVID-19 infection.
Clinical parameters during hospitalization
The echocardiographic parameters revealed that COVID +ve patients had significantly lower ejection fractions and higher WMSI, while severe MR and TAPSE were worse in COVID +ve patients but did not reach statistical significance (Table II).
Table II
Clinical parameters during hospitalization
[i] Data are mean ± SD, median (range), or number (%) of patients. LAD – left anterior descending, TIMI – thrombolysis in myocardial infarction, EF – left ventricular ejection fraction, WMSI – wall motion score index, MR – mitral regurgitation, TAPSE – tricuspid annular plane systolic excursion, BB – β-blockers, ACEi – angiotensin-converting enzyme inhibitors, ARBs – angiotensin receptor blockers, OACs – oral anticoagulants, AF – atrial fibrillation.
COVID +ve patients less frequently received β-blockers during hospital admission, while inotropes and oral anticoagulation were significantly needed in this group. The addition of other medications such as renin-angiotensin-aldosterone system (RAAS) inhibitors, diuretics, amiodaron, nitroglycerin, and ivabradine did not show a significant difference between the groups. Also, despite being worse in COVID +ve patients, differences in ST-segment resolution and TIMI risk score did not reach statistical significance.
Comparing in-hospital outcomes between the two groups, more extended hospital stay, in-hospital mortality, cardiogenic shock, malignant arrhythmias, and the need for intubation and mechanical ventilation were significantly higher in COVID +ve patients while developing heart failure, atrial fibrillation, and bradyarrhythmias were also higher in COVID +ve patients but not to the level of statistical significance.
Six-month clinical outcomes
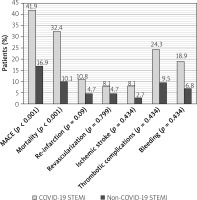
Compared to COVID -ve patients, patients who tested positive for COVID-19 showed significantly higher MACE rates, mortality, thrombotic complications, and bleeding at 6 months follow-up. On the other hand, reinfarction, the need for repeated revascularization, and ischemic stroke did not show a statistically significant difference between the groups (Figure 2).
Independent predictors of 6-month mortality
Multivariable stepwise logistic regression analysis identified STR (OR = 8.633; 95% CI: 2.652–28.103, p < 0.001), lower systolic blood pressure (OR = 1.106 per 1 mm Hg decrease; 95% CI: 1.045–1.171, p = 0.001), presence of severe MR (OR = 7.728; 95% CI: 1.834–32.569, p = 0.005), admission Killip class > 1 (OR = 7.469; 95% CI: 1.741–32.046, p = 0.007), occurrence of AF (OR = 5.718; 95% CI: 1.213–26.962, p = 0.028), and anterior STEMI (OR = 3.090; 95% CI: 1.046–9.129, p = 0.041) as significant and independent predictors of 6-month mortality in STEMI patients during the COVID pandemic (Table III).
Table III
Multivariable logistic regression analysis of 6-month mortality (independent predictors)
Discussion
In the current study, we evaluated a total of 222 STEMI patients, 74 COVID-positive (group I) and 148 COVID-negative (group II), for a mean follow-up of 6 months, and we identified the impact of COVID-19 superinfection on those high-risk groups of patients. This study differs from previous studies in that it included all consecutive STEMI patients who presented to our hospital, and we extended the follow-up beyond the in-hospital outcomes to a relatively long period. We also applied propensity score (PS) matching to yield two groups in a 1 : 2 ratio to strengthen the study’s results. Additionally, we identified the predictors of 6-month mortality in STEMI patients during the COVID-19 pandemic, and we shed light on the increased incidence of thrombotic complications in the COVID-positive patients that may change the management strategy of those high-risk groups of patients.
Baseline characteristics of study cohort
There was no statistically significant difference between the groups regarding age, gender, or other risk factors, which is consistent with a previous study conducted by Barbero et al. in which they looked for acute coronary syndrome patterns during the COVID-19 outbreak and gender differences [16]. In this study, although the reduction of MI hospital admission was consistent between men and women, the significant increase in women presenting with dyspnea caused more treatment delays.
In our study, the main presenting symptom of patients in group I was predominantly chest pain, contrary to patients in group II, who had more angina equivalent symptoms at their presentation, making a statistically significant difference between the groups.
A study in Turkey of the COVID-19 outbreak and its impact on patients with ST-segment elevation also noted similar results in which chest pain was the main presenting symptom of STEMI in the pre-COVID era, whereas dyspnea and other angina equivalent symptoms were the main presenting symptoms in the COVID era [17].
In a neural network model analysis of data collected from 161 catheterization laboratories in Poland throughout the year 2021, the most crucial variable for the prognosis of STEMI patients was the time from first medical contact to balloon inflation [18]. In the present study, there was no statistically significant difference between groups as regards the door-to-balloon or the total ischemic time. This could be explained by applying the same triage for all the patients in both groups at their presentation to the hospital, as these high-risk patients were considered COVID +ve until proven otherwise. Our protocol is consistent with a previous protocol that considered patients as COVID-19 infected and used airborne PPE while awaiting swab results in order to provide timely treatment; then, after revascularization, the COVID-19 status should be assessed, and if the infection is proven, dedicated isolation in a coronary care unit or ward should be organized [1].
The Killip class and the mean heart rate (HR) at presentation were significantly higher in group I, which could be explained by combined pulmonary and cardiac involvement. A study of acute myocardial infarction patients before COVID-19 infection versus during the pandemic and their 6-month outcomes revealed no statistically significant difference in Killip class in STEMI patients between pandemic and pre-pandemic eras [19]. This controversy could be clarified by the retrospective design of this study and by the small number of STEMI patients included.
At the beginning of the epidemic, the early Chinese algorithms sacrificed primary PCI in favor of protecting healthcare personnel, and immediate fibrinolysis after a rapid COVID-19 test was their strategy [1]. European societies recommend a halfway approach as they consider that the first-line therapeutic approach to STEMI should not be changed by COVID-19 infection, but they also reported a substantial decrease in the total number of primary PCI during the pandemic [20, 21]. Our study was not performed at the beginning of the epidemic, which explains the absence of significant difference between the groups regarding reperfusion strategy.
In-hospital management and clinical outcomes
Regarding the echocardiographic parameters during the hospital stay, WMSI and LV ejection fraction were worse in group I. In a study of the COVID-19 pandemic effect on the presentation and hospital outcomes of patients with STEMI, a significantly lower left ventricular ejection fraction was observed in STEMI patients during the COVID era [22].
Although some studies showed the beneficial effect of the use of β-blockers (BB) to antagonize the COVID-19-related hyperinflammatory response by reducing the circulating cytokines [23, 24], only 41.9% of STEMI patients with positive COVID-19 infection were given BB in the present study due to the prevalence of cardiogenic shock in this group of patients.
The current guidelines did not consider COVID-19 infection per se as an indication of prescribing a therapeutic dose of oral anticoagulants (OACs). They stated that a prophylactic dose of anticoagulation, preferably with low molecular weight heparin (LMWH), is indicated in hospitalized COVID-19 patients, making the therapeutic dose of OACs only if there is a compelling cardiac condition [25]. However, venous thromboembolism (VTE) is a serious threat in severe COVID infection. In a review of COVID-19 and thrombotic complications, the authors found that the appropriate dose of prophylactic anticoagulation did not prevent the increase of deep venous thrombosis (DVT) [26]. Also, prophylactic anticoagulation did not prevent the occurrence of pulmonary embolism as evident in a study of 184 consecutive ICU patients with COVID-19 infection. A confirmed VTE was demonstrated in 27% of patients by CT pulmonary angiogram [27]. In our study, the COVID group were routinely prescribed prophylactic dose of anticoagulation which did not prevent the development of VTE in a significant number of patients that required a shift to OACs at a therapeutic dose.
Cardiogenic shock, malignant arrhythmias, the need for intubation with mechanical ventilation, and mortality were significantly higher in group I. Heart failure was higher in group I, but it did not reach statistical significance. In a retrospectively designed study of the in-hospital outcomes of STEMI patients with COVID infection, Ayad et al. found that STEMI patients with COVID-19 infection had a higher incidence of in-hospital mortality mainly because of arrhythmia (ventricular fibrillation) and intractable cardiogenic shock. Also in-hospital development of heart failure was higher in STEMI patients with COVID-19 than STEMI patients without COVID-19, although the difference was not statistically significant [28]. In addition, in another study of COVID-19 infected STEMI patients and their in-hospital outcomes, patients with COVID-19 had increased in-hospital mortality driven by non-cardiovascular and cardiovascular causes [29]. In the same study, MACE, cardiogenic shock, and mechanical ventilation after PCI were more frequent in the COVID-19 group, making these findings consistent with ours.
Six-month follow up
Most studies of STEMI patients in the COVID-19 era either used a retrospective design or looked for outcomes during the hospital stay only. Kaziród-Wolski et al. reported an increase in periprocedural morbidity and mortality in COVID +ve patients in a total of 47,940 patients treated for ACS in 2020 [30]. In their study, the occurrence of no-reflow and the use of glycoprotein IIb/IIIa inhibitors significantly and independently impacted periprocedural mortality in COVID +ve patients, implying the development of a thrombotic process associated with COVID infection. In our study, we aimed to reveal the impact of COVID-19 infection in this critical group over 6 months of follow-up in order to highlight the implications of COVID-19 infections for the patients even beyond their hospital stay. We found that after 6 months of follow-up, MACE and mortality were significantly higher in the COVID-19 group. Our results also revealed that both thrombotic and bleeding complications were significantly higher in group I, keeping in mind that those patients had a higher percentage of OAC intake. In fact, this result emphasizes the complicated effect of COVID-19 on these critical patients.
In our study, the independent predictors of 6-month mortality were STR on ECG, lower systolic blood pressure and Killip class > 1, developing AF or severe MR during admission, and anterior location of STEMI. Our study found STR to be an independent and significant predictor of mortality after 6 months (p < 0.001). In a previous study that aimed to use baseline electrocardiographic parameters as predictors of impaired LV systolic function in patients who underwent PCI due to first-time STEMI, the authors noted that patients with complete (≥ 70%) resolution of ST-segment elevation had lower 1–3-year mortality and lower rates of cardiovascular adverse events, and had better preservation of left ventricular function in comparison with partial (30–70%) or no (< 30%) ST-segment resolution [31].
A study of acute myocardial infarction patients and the outcomes of in-hospital development of AF revealed that patients who developed in-hospital AF had higher in-hospital mortality and long-term mortality at 85 months of follow-up. However, the differences did not reach statistical significance [32]. Also, in the study mentioned above, AF was not found to be an independent predictor of mortality; comparing their findings to ours highlights the effect of the COVID pandemic on the impact of AF development on mortality.
Study limitations
Our study had some limitations. First, it is a single-center study with a relatively small number of COVID-19 patients included, which may have affected the statistical power to detect significant differences. Although we applied PS matching to select a reasonably similar cohort of COVID –ve STEMI patients in terms of baseline characteristics, the effects of some unmeasured confounders cannot be removed entirely. We highlighted the increased incidence of thrombotic complications in COVID-19 patients, but we did not perform a subgroup analysis of the optimal treatment strategy for those patients, especially regarding the routine prescription of OACs. In addition, the 6-month follow-up period is relatively short, and extended follow-up should be performed in further studies.
Conclusions
Our study showed that STEMI patients with superimposed COVID-19 infection had worse clinical outcomes with a three-fold increase in in-hospital mortality with a more complicated course during hospitalization requiring a prolonged hospital stay. During the 6-month follow-up, those patients also had increased rates of MACEs with an increase in both thrombotic and bleeding complications, which necessitates further studies to provide strong evidence about the ideal management strategy.