Introduction
Although pain after laparoscopic cholecystectomy (LC) is less intense than that after open cholecystectomy, some patients still experience considerable discomfort during the first 24 h. Pain after LC is still the main complaint which prolongs hospital stay [1]. Opioids are one of the choices for perioperative analgesia [2]. However, the use of opioids may cause excessive sedation and induce respiratory depression. Many patients may experience nausea and vomiting [3]. All of these may lower the benefits of analgesia. Non-opioid drugs were recommended to be used first to decrease the number of opioids after abdominal surgery [4]. Therefore, we need to study and evaluate newer non-opioid pain medications for an opioid-reduction strategy.
Dexmedetomidine (DEX) is widely used to provide sedation, analgesia, and sympatholysis [5, 6]. Previous studies show that DEX may be a potential non-opioid pain medication in the perioperative period and decrease the opioid consumption and opioid-associated adverse events [7, 8]. Some randomized controlled trials have investigated DEX use in patients undergoing LC, but evidence on DEX for postoperative pain scores and opioid consumption remains unclear due to the small sample sizes.
Aim
We performed this meta-analysis to evaluate the DEX use for the opioid consumption and pain control after LC.
Material and methods
Search strategy and study criteria
We carried out this meta-analysis following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) guidelines [9] and no ethical approval was required. A systematic literature search of RCTs was conducted from 1999 to March 2019 in PubMed, EMBASE, and Cochrane Library. The search strategy included the combination of the keywords: “dexmedetomidine”, and “cholecystectomy”, or “laparoscopic cholecystectomy”, or “LC”, and “ gallbladder”, or “ cholecyst”, or “ cholecystitis”.
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) randomized controlled trials only, and as an original article, (2) studies published in English, (3) trials compared the clinical indicators between DEX and placebo or other drugs.
We excluded studies that (1) were expert consensuses, reviews, case reports, letters to the editor or retrospective studies, (2) articles without the full text, (3) were performed by open operations, and (4) lacked clinical outcome data and it was not possible to contact the authors.
Data extraction
The data of eligible studies were extracted independently by two investigators (GMZ and PL). The following contents were collected: age, gender, weight, the time of surgery and anaesthesia, and the method of DEX application. We solved disagreements through discussion for consensus and considered the PubMed database in preference. The authors used the Cochrane risk of bias tool and Jadad scale to assess the quality of the eligible studies.
Outcomes
Opioid consumption in the first 24 h after the operation, the time of first request of analgesia, visual analogue scale (VAS) scores in the 24 h after the operation, the incidence of patients’ need for rescue analgesics, opioid-related adverse effects, DEX-related adverse effects and other complications
Statistical analysis
We used odds ratios (OR) with 95% confidence intervals (95% CI) in all analyses for dichotomous variable (reported with incidence). The statistical method of Hozo et al. [10] and weighted mean difference (WMD) with 95% CI were used for continuous variable (reported as mean ± standard deviation, median and interquartile range, or median and range). The inconsistency statistic (I 2) was calculated to assess the heterogeneity. A random effect model was suitable for high heterogeneity (I 2 ≥ 50%), and a fixed effects model for low heterogeneity (I 2 < 50%). Begg’s and Egger’s funnel plot analysis were conducted to evaluate publication bias. Sensitivity analysis, meta-regression and subgroup analysis were performed to explore possible heterogeneity when necessary with significance defined as p < 0.1. All statistical analysis was performed in REVMAN (version 5.0; Cochrane Collaboration, Oxford, UK), SAS (version 9.4; SAS Institute Inc) and Stata (version 9.0; StataCorp LP), and the significance was defined as p < 0.05, except where specially mentioned.
Results
Study characteristics
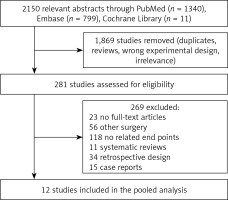
Selection of the randomized controlled trials for this meta-analysis is shown in Figure 1. Fourteen aspects of twelve trials enrolling 967 patients were subjected to analysis (Figure 1) [11–22]. Eight trials used placebo as a control [11–13, 15–18, 20], whereas three used paracetamol [14, 21, 22], one used dexamethasone [19], and one used clonidine or tramadol [16]. DEX infusion commenced at a rate of 0.05 to 0.6 µg/kg/h in 9 studies [11, 12, 14, 15, 17, 18, 20–22]. Among these, patients received DEX with a loading dose of 0.5 or 1 µg/kg in 6 studies [11, 14, 17, 20–22]. DEX was infused at a loading dose of 0.5 or 1 µg/kg in another 3 studies [13, 16, 19].
For outcomes, opioid consumption in the first 24 h after the operation was reported in eight trials [11–15, 19–21], the time of first request of analgesia was reported in seven aspects of five trials [12–14, 16, 19], VAS scores 24 h after the operation was reported in six trial [12, 13, 17, 18, 21, 22], and the incidence of patients’ need for rescue analgesics was reported in six aspects of four trials [15, 16, 18, 20].
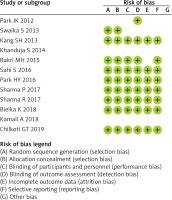
Tables I and II show the general characteristics of the included studies. Table III and Figure 2 summarize the quality scores.
Table I
General design of studies included in this meta-analysis
Table II
General characteristics of patients included in each study
Table III
Quality scores of studies included in this meta-analysis
Effect of DEX on opioid consumption in first 24 h after operation
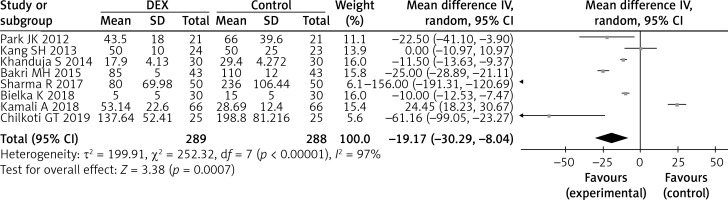
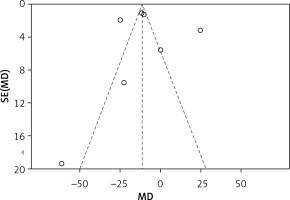
The opioid consumption in the first 24 h after the operation was investigated in 577 enrolled participants and was significantly reduced by DEX (eight studies; WMD = –19.17; 95% CI: –30.29 to –8.04; p = 0.0007; I 2 = 97%; Figure 3). No significant publication bias existed (Begg’s test, p = 0.27; Egger’s test, p = 0.43; Figure 4).
A subgroup analysis was conducted to explore heterogeneity for the primary outcome, and there were eight groups according to different characteristics as shown in Table IV. Significant heterogeneity was found in the subgroups of patients grouped by age, male proportion, administration timing (before induction versus after induction), and Jadad score. No heterogeneity was detected for opioid consumption in the first 24 h after the operation in other subgroups (Table IV).
Table IV
Subgroup analysis for heterogeneity of primary outcome
Table V presents the results of a meta-regression analysis. No significant differences for opioid consumption in the first 24 h after the operation were found.
Table V
Meta-regression analysis for heterogeneity of primary outcome
A sensitivity analysis was conducted and showed that all studies had the same opioid reduction effect (p < 0.05) except Sharma R [14].
Effect of DEX on the time of the first request of analgesia
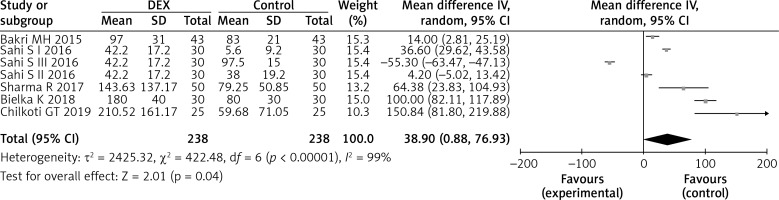
The time of the first request of analgesia was reported in 476 study participants, and DEX infusion significantly prolonged the time of the first request of analgesia (five studies; WMD = 38.90; 95% CI: 0.88–76.93; p = 0.04; I 2 = 99%; Figure 5).
Effect of DEX on VAS scores 24 h after operation
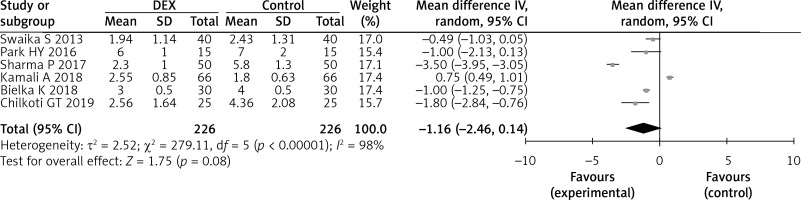
VAS scores 24 h after the operation were reported in 452 study participants and were lower with DEX use, but the difference was not statistically significant (six studies; WMD = –1.16; 95% CI: –2.46 to 0.14; p = 0.08; I 2 = 98%; Figure 6).
Effect of DEX on incidence of patients’ need for rescue analgesics
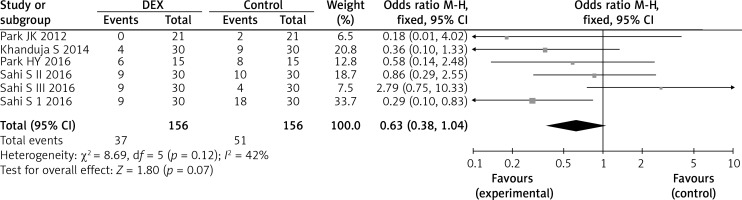
The occurrence of patients’ need for rescue analgesics was reported in 312 study participants and was lower with DEX use, but the difference was not statistically significant (four studies; OR = 0.63; 95% CI: 0.38–1.04; p = 0.07; I 2 = 42%; Figure 7).
Effect of DEX on opioid related-adverse events
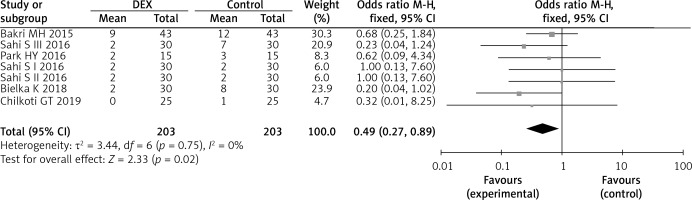
Opioid related-adverse events were reported in five studies [12, 13, 16, 18, 19]. Among these, post-operative nausea or vomiting (PONV) was reported in seven aspects of five trials enrolling 406 study participants and was significantly reduced by DEX (five studies; OR = 0.49; 95% CI: 0.27–0.89; p = 0.02; I 2 = 0%; Figure 8). Pruritus was only reported in one study [12], and there was no statistically significant difference between groups (p = 0.28).
DEX related adverse events
Only one trial reported DEX related adverse events [12]. No differences were found in the incidences of hypotension and bradycardia.
Other outcomes
The effect of DEX on the duration of stay in the post-anaesthesia care unit (PACU) was explored in one study [17], which showed that DEX shortened the PACU stay in the control group than in the DEX group (61.4 ±5.7 min vs. 69.7 ±14.1 min, respectively, p = 0.001). The effect of DEX on the mean extubation time was reported in two studies [12, 18]. Our meta-analysis showed that there was no statistically significant difference in the mean extubation time owing to the DEX use (two studies; WMD = –5.69; 95% CI: –14.22 to 2.83; p = 0.19; I 2 = 98%).
Discussion
Our meta-analysis suggested that compared with the control intervention, DEX use significantly reduced postoperative opioid consumption, improved the duration of the analgesic effect, and lowered the incidence of PONV during LC.
DEX has been demonstrated to be effective for improved analgesia and may be an optimal drug for pain relief effects [23]. A meta-analysis performed by Schnabel reported that DEX infusion relieved postoperative pain and reduced opioid consumption in various elective surgeries [24]. Another meta-analysis by Le Bot showed a similar reducing effect of DEX for opioid, postoperative pain and PONV in multiple types of elective surgery [25]. There were studies focused on the efficacy of DEX in LC, but the conclusions are conflicting. Our study was the first meta-analysis to evaluate the efficiency of DEX for opioid consumption and pain control and indicated that intravenous DEX significantly decreased postoperative opioid consumption for adult patients undergoing LC.
Age-related reduction in renal and hepatic function may decrease the systemic clearance of opioids. Among older adults, we should start with the lower available dose of opioids compared with their younger counterparts [26]. A recent article reported that opioid metabolism differed according to gender due to the difference of the inhibitory circuit modulated by gonadal steroids [27]. Opioid use is more effective in males, so these sex differences must be considered in pain management [28]. In this meta-analysis, opioid consumption in the first 24 h after the operation was reduced in the subgroup of younger age (< 45 years) or lower male proportion (< 30%).
Opioid consumption was regularly used for pain relief after surgery. However, opioid associated adverse effects must be taken into account. In our study, the incidence of PONV was reduced in the DEX group, which was consistent with previous studies [29, 30].
There are several limitations to our study: (1) There were only twelve RCTs with 976 patients in our study. More RCTs with higher quality will be helpful for future study; (2) There was a tremendous amount of clinical heterogeneity between studies. Some important data were not reported, so these may influence the outcomes; (3) Although subgroup analysis, meta-regression analysis and sensitivity analysis were performed, heterogeneity still existed due to design differences of included RCTs. (4) The enormous heterogeneity of included studies with a relatively small sample size may result in a great statistical bias for the observed effects.
In conclusion, our study indicates that DEX use significantly improves the duration of the analgesic effect and reduces postoperative opioid consumption after LC. Moreover, there is less opioid-related PONV as a result of DEX use.