Summary
There is evidence to suggest that the prognosis of non-ST-segment elevation myocardial infarction with multivessel disease is worse than that of patients with single-vessel disease. However, the optimal revascularization strategies including immediate complete revascularization (ICR), staged complete revascularization during index PCI (SCR), and planned complete multi-vessel percutaneous coronary intervention during (PCI) during a second hospitalization (MV-PCI) for these patients remain unclear. In this network meta-analysis of 8 studies involving nearly 34,151 participants evaluating the impact of different revascularization strategies on clinical outcomes, we found that MV-PCI was associated with a reduced risk of major adverse cardiovascular events (OR = 0.53), all-cause mortality (OR = 0.53), cardiovascular mortality (OR = 0.48), and repeat revascularization (OR = 0.55). Furthermore, a cluster ranking plot demonstrated that MV-PCI had the best efficacy (all-cause mortality) and safety (recurrent myocardial infarction), while culprit-only revascularization (COR) had poorer efficacy and safety. It is the first analysis that not only investigates culprit-only revascularization and multivessel revascularization but also examines different approaches to complete revascularization based on multivessel disease, including ICR, SCR and MV-PCI. And for the first time, it evaluates the impact of these four different revascularization approaches on prognosis (confirming the superiority of MV-PCI), which was not previously analyzed. The analysis also indicates that regardless of the type of multivessel revascularization approach, the prognosis was better than that of COR, which, in fact, undermines the conventional practice of using COR in the interventional therapy of such patients
Introduction
Multivessel disease (MVD) identified on coronary angiography is a known independent predictor of worse prognosis in patients with ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI) [1–3]. This has led to the dilemma of which revascularization strategy to choose – coronary artery revascularization only of the culprit-only revascularization (COR), immediate complete revascularization (ICR), staged complete revascularization during index PCI (SCR), or planned complete multi-vessel percutaneous coronary intervention during a second hospitalization (MV-PCI). In non-ST-segment elevation myocardial infarction (NSTEMI) patients, the role of complete PCI revascularization remains unclear [4]. Due to insufficient evidence from clinical trials, guidelines and expert opinions also fail to provide guidance [5–7]. As a result, neither American nor European guidelines make definitive recommendations on which coronary revascularization strategy should be considered for these patients [8, 9].
With the increasing use of high-sensitivity troponin in the assessment of acute chest pain, the detection rate of NSTEMI is on the rise. Epidemiologic data suggest that NSTEMI is more common compared to STEMI, and approximately 40–60% of such patients undergoing coronary angiography (CAG) are found to have multivessel disease, with poorer prognosis compared to single vessel disease (SVD) NSTEMI patients and double the annual mortality rate [10, 11]. Revascularization strategies have evolved over time, and the recent COMPLETE trial appeared to favor upfront multivessel PCI (MV-PCI) in STEMI patients [12]. Whether this can be extrapolated to NSTEMI patients remains controversial.
Previous reviews and meta-analyses focused only on head-to-head comparisons of different revascularization modalities while overlooking comparison across multiple revascularization strategies. However, studies on revascularization strategies in NSTEMI patients with MVD are scarce, with only one randomized controlled trial, the SMILE trial, comparing upfront versus staged multivessel revascularization in these patients [13]. Therefore, in this study, we pooled data across different revascularization strategies to analyze adverse outcomes such as major adverse cardiovascular events (MACEs), all-cause mortality, recurrent myocardial infarction, and repeat revascularization, which previous meta-analyses on this topic did not provide.
Hence, there is a need to perform a network meta-analysis of these studies to update the data and thereby assess the impact of various revascularization modalities on clinical outcomes in NSTEMI patients with MVD.
Methods
This network meta-analysis report adheres to the PRISMA 2020 statement [14]. As this study only contained previously published study data, ethical approval was not required. This study was registered with PROSPERO (CRD42024516284).
Search strategy
Electronic searches of PubMed, Embase, the Cochrane Library, and Web of Science databases were conducted from inception to October 3, 2023 without any language restrictions, using search terms including but not limited to “non-ST elevated myocardial infarction”, “multivessel disease”, and “revascularization strategies”.
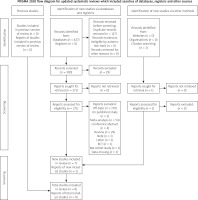
No time or language limitations were used. Additionally, reference lists of published trials, meta-analyses, and review articles were hand searched comprehensively to identify any other papers not detected initially from the systematic searches. Articles were first shortlisted based on the title and abstract and then full texts of articles were reviewed to select studies. Articles retrieved from the systematic search were exported to EndNote Reference Library (version x7.5; Clarivate Analytics, Philadelphia, Pennsylvania) software. In this software, duplicate articles were searched for and deleted. The remaining articles were extracted by the same two authors (T.T.C. and C.L.) using a standardized extraction form. A third reviewer (J.L.M) was then consulted to resolve any disagreements in opinion. The study flow diagram is shown in Figure 1.
Inclusion and exclusion criteria
We selected all observational cohort studies that assessed the clinical impact of different revascularization strategies in NSTEMI. The selected population met the following criteria: 1) adults (≥ 18 years) with NSTEMI and MVD; 2) the study included at least a culprit-only vessel group and a multivessel group (at least one more non-culprit vessel treated in addition to the culprit vessel); 3) literature study endpoints should contain metrics of interest to be observed. The definition of NSTEMI followed the Fourth Universal Definition of MI [15]. Angiographic MVD was defined as ≥ 50% stenosis in at least two major epicardial coronaries or their major branches. Articles were excluded for the following reasons: 1) STEMI, 2) non-topical, 3) meta-analysis, 4) conference abstract, 5) review, 6) note, 7) letter, 8) non-cohort study, 9) randomized controlled trial (RCT), 10) missing data, 11) unpublished study. If data from the same trial were reported in multiple publications, the article with the longest follow-up time was chosen. Disagreements were resolved by consensus.
Patients were divided into 4 groups according to PCI strategy: A: “Culprit-only revascularization” (COR) refers to intervention limited only to IRA lesion, with all other lesions left untreated; B: “Immediate complete revascularization” (ICR) refers to treatment of both culprit and non-culprit arteries during index PCI; C: “Staged complete revascularization” (SCR) refers to patients who underwent PCI for non-infarct-related arteries (non-IRA) during index PCI for IRA or staged PCI for non-IRA during index hospitalization, but this review did not include NSTEMI and MVD patients who underwent staged PCI after discharge. D: “Multivessel PCI” (MV-PCI) was defined as staged PCI for non-IRA within 60 days, but we could not exclude the effect of “immediate complete revascularization”. The bystander vessel was treated during planned secondary hospitalizations [10, 11, 16–26].
Data extraction and quality assessment
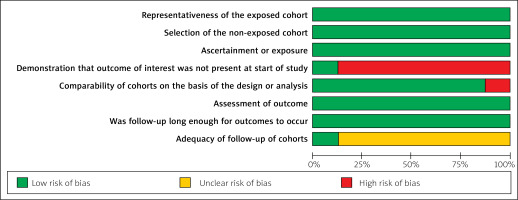
Relevant data were extracted independently by two reviewers (T.T.C. and C.L.). The data included population, study and patient characteristics of interest, and relevant outcomes. Outcomes of interest relevant to efficacy and safety of revascularization strategies were extracted. The primary efficacy outcome was all-cause mortality. Secondary outcomes of interest were MACE events, cardiovascular mortality, and repeat revascularization. Safety of the different strategies was assessed by evaluating rates of recurrent myocardial infarction. The quality of included studies was assessed by two authors (X.L. and T.W.) using the Newcastle-Ottawa Scale tool [27]. This was supplemented by assessments of study selection, comparability of the two cohorts, and outcome assessment (Table I). Any disagreements regarding study selection, data extraction, and quality assessment were resolved through consensus or by a third reviewer (J.L.M.).
Table I
Quality assessment via Newcastle-Ottawa Scale
Statistical analysis
All statistical analyses were performed using Stata MP manager (version 15.1), RevMan 5.4, and ADDIS 1.16.5.
Network meta-analyses were then performed to compare different revascularization strategies simultaneously for each outcome using a frequentist framework. A network plot was drawn to describe the geometry of the treatment network. Consistency of the treatment network was evaluated by comparing direct and indirect effect estimates for the same comparison. Node splitting analysis was also performed to assess inconsistency.
Effect sizes for network meta-analyses were presented as odds ratios (ORs) with 95% confidence intervals (CI). League tables were constructed to illustrate the relative treatment effects of all comparisons in the network. Ranking probabilities of each intervention were calculated to provide the hierarchy of treatments. The extent of uncertainty for all effect sizes was assessed using prediction intervals. Sensitivity analyses were conducted by excluding studies one at a time to evaluate the robustness of findings.
Results
Study selection and characteristics
From the 428 studies identified in our retrieval, 127 duplicates were removed and 271 titles and abstracts were screened for eligibility. Of these articles, 264 were excluded, primarily because they were not topical (n = 170 articles), unpublished studies (n = 2), non-cohort studies and RCTs (n = 2), reviews and meta-analyses (n = 83), and other reasons (n = 9), leaving 7 eligible studies included in our review. A total of 8 studies involving 34,151 patients [10, 11, 21–26] were included in the quantitative analysis and assessed in the network meta-analysis; of these, 15,625 received COR = 3,563 received SCR, 13,276 received ICR, and 1,687 received MV-PCI. The characteristics of the individual selected studies and baseline characteristics of the patients are listed in Tables II and III.
Table II
Baseline characteristics of patients
Table III
Description of included studies
Quality assessment and publication bias
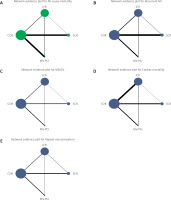
The risk of bias for the observational cohort studies collected for this pooled analysis was low. The quality of observational studies was screened using the Newcastle-Ottawa Scale (Figure 2). There was no masking of any subjective assessments in the studies. Inconsistency tests for all outcome metrics found p > 0.05 for all comparisons of treatment regimens, indicating good consistency between direct and indirect evidence for all outcome metrics. Further local inconsistency was tested using the node splitting method, which found p > 0.05 for all comparisons, indicating no local inconsistency and greater authenticity of network meta-analysis results. Inconsistency tests forming closed loops for all outcome metrics found IF close to 0 and CI including 0 for all, suggesting good consistency between direct and indirect comparison results within closed loops and greater authenticity of network meta-analysis. Network plots of all eligible comparisons for primary and secondary outcomes are shown in Figure 3. Pooled ORs and 95% CIs for efficacy and safety of different surgical strategies in the network meta-analysis are depicted in Figure 4. Network plots of all comparators are introduced in Figure 5. Figures 6 A–E lists comparisons of all treatment regimens for all outcomes. Comparison-adjusted funnel plots were used to analyze publication bias [28], and the comparison-adjusted funnel plots did not show signs of asymmetry.
Figure 3
Network of eligible comparisons for primary efficacy and safety outcomes. A – All-cause mortality. B – Recurrent MI. The size of the node corresponds to the number of individual studies that studied the interventions. The directly compared interventions are linked with a line, the thickness of which corresponds to the number of studies that assessed the comparison
SCR – staged complete revascularization, ICR – immediate complete revascularization, COR – culprit-only revascularization, MV-PCI – Multi-vessel PCI, MI – myocardial infarction.
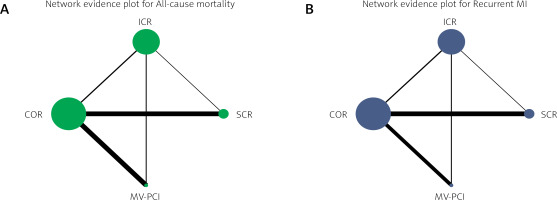
Figure 4
Network meta-analysis of primary efficacy (all-cause mortality) and safety (recurrent MI) outcomes. Interventions are ordered by ranking for all-cause mortality. Results are the OR (95% CI) from the network meta-analysis between the column-defining intervention and row-defining intervention. Comparisons should be read from left to right. Numbers in bold represent statistically significant results
SCR – staged complete revascularization, ICR – immediate complete revascularization, COR – culprit-only revascularization, MV-PCI – multi-vessel PCI, MI – myocardial infarction, OR – odds ratio.
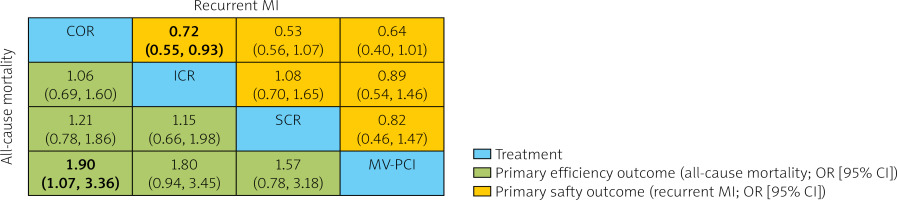
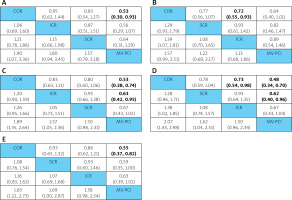
Figure 6
A – Network estimated odd ratios (95% confidence intervals) of treatment options for all-cause mortality. B – Network estimated odd ratios (95% confidence intervals) of treatment options for recurrent MI. C – Network estimated odd ratios (95% confidence intervals) of treatment options for MACEs. D – Network estimated odd ratios (95% confidence intervals) of treatment options for cardiac mortality. E – Network estimated odd ratios (95% confidence intervals) of treatment options for repeat revascularization
SCR – staged complete revascularization, ICR – immediate complete revascularization, COR – culprit-only revascularization, MV-PCI – multi-vessel PCI, MACEs – major adverse cardiac and cardiovascular events, MI – myocardial infarction. *Treatments are ordered by SUCRA rank. Comparisons between treatments should be read from column to row for each outcome (row treatment is reference)
Results of network meta-analysis
MACEs
The definition of MACEs (major adverse cardiovascular events) varied across studies. In our network meta-analysis, we only considered studies that used MACEs as a composite endpoint of all-cause mortality, cardiovascular mortality, recurrent myocardial infarction, and repeat revascularization. A total of 2738 patients’ data from the selected 8 studies reported MACE outcomes. As shown in Figure 6 C, MV-PCI had significantly lower risk of MACEs compared to COR (OR = 0.53, 95% CI [0.38–0.74], surface under the cumulative ranking curve (SUCRA) value: 94.6%) and ICR (OR = 0.63, 95% CI [0.42–0.95], SUCRA value: 56.6%). According to the comparison-adjusted funnel plots, no significant publication bias was detected in this outcome indicator.
All-cause mortality
Among the selected 8 studies, 5 studies containing data on 1222 reported all-cause deaths were included. Our pooled analysis in Figure 6 A showed that COR had significantly higher all-cause mortality compared to MV-PCI (OR = 0.53, 95% CI [0.30–0.93], SUCRA value: 5.4%), with no significant differences between other regimens. According to the comparison-adjusted funnel plots, no significant publication bias was detected in this outcome indicator.
Cardiac mortality
Among the selected 8 studies, 7 studies containing data on 583 patients reported results for cardiac mortality. Our pooled analysis in Figure 6 D showed that COR had significantly higher cardiac mortality compared to ICR (OR = 0.73, 95% CI [0.54–0.98], SUCRA value: 44.2%) and MV-PCI (OR = 0.48, 95% CI [0.34–0.70], SUCRA value: 1.8%), and SCR had significantly higher cardiac mortality compared to MV-PCI (OR = 0.62, 95% CI [0.40–0.96], SUCRA value: 1.8%). According to the comparison-adjusted funnel plots, no significant publication bias was detected in this outcome indicator.
Recurrent MI
Among the selected 8 studies, 7 studies containing data on 465 patients reported outcomes for recurrent myocardial infarction. Our pooled analysis in Figure 6 B showed that ICR had significantly lower recurrent myocardial infarction occurrence compared to COR (OR = 0.72, 95% CI [0.55–0.93] SUCRA value: 96.8%). According to the comparison-adjusted funnel plots, no significant publication bias was detected in this outcome indicator.
Repeat revascularization
All studies reported data on 1013 patients for our analysis of repeat revascularization. Our pooled analysis in Figure 6 E showed that MV-PCI patients had significantly lower repeat revascularization rates compared to COR (OR = 0.55, 95% CI [0.37–0.82], SUCRA value: 82.0%). According to the comparison-adjusted funnel plots, no significant publication bias was detected in this outcome indicator.
Discussion
Whether performing PCI for non-culprit lesions in patients with multivessel coronary artery disease presenting as NSTEMI can improve long-term prognosis remains unclear, with discordant results from clinical studies [29, 30]. This study utilized network meta-analysis (NMA) to compare the efficacy and safety of 4 revascularization strategies currently used for NSTEMI with MVD, including a total of 8 observational studies and 34,151 patients, making it the most comprehensive and robust NMA on this topic to date to provide evidence for different revascularization strategies in MVD patients presenting with NSTEMI. The results indicate that MV-PCI is one of the most efficacious treatments, with acceptable all-cause mortality and recurrent myocardial infarction rates.
Most previous meta-analyses only included STEMI or NSTE-ACS, while our study included a large number of observational studies with longer follow-up to address discrepancies and limitations in the literature, enhancing the generalizability and reliability of our results. Compared to other studies, the effect of non-NSTEMI was excluded, and outcome metrics analyzed were more comprehensive, including both efficacy and safety metrics, leading to more accurate results.
Earlier studies reported that in NSTEMI patients with MVD, conventional multivessel PCI was not superior to culprit-only PCI: and complete revascularization did not seem to provide significant clinical benefits over single-vessel PCI [31, 32]. As a result, ACC/AHA and ESC guidelines have not been proactive about revascularization of non-culprit lesions over the years (class II recommendation) [8, 33–35]. The reasons may be related to the complexity and severity of lesion vessels in NSTEMI patients with MVD, where complete revascularization increases contrast use and lengthens procedure time, leading to poorer long-term prognosis. The ESC guidelines for coronary revascularization did not provide recommendations on timing of non-culprit vessel intervention, noting that selection of the revascularization strategy should be decided after overall assessment of patient condition, while advising non-IRA treatment decisions be made in consultation with the heart team [33]. Therefore, providing the optimal revascularization strategy after weighing risks is critical for patients. However, several subsequent studies have reported favorable outcomes with complete revascularization, whether performed during the index procedure or staged in NSTEMI [13, 30, 36]. These benefits were driven primarily by reductions in myocardial infarction. The results of this analysis further confirm that in NSTEMI patients with MVD, upfront multivessel complete revascularization may be a better treatment strategy compared to COR. Although our analysis cannot elucidate the exact reasons why multivessel intervention improves clinical outcomes, it may be related to the following: multivessel intervention helps not only reperfusion of the culprit artery but may also aid in improving blood flow in non-culprit vessels, improving left ventricular contraction and the subsequent prognosis [37].
Our study showed that MV-PCI had fewer MACE events compared to COR and ICR, and lower cardiac mortality compared to SCR. MV-PCI led to reductions in the MACE composite endpoint compared to COR whether complete revascularization was performed upfront or culprit-only during the index procedure. In assessing outcome metrics, the MV-PCI revascularization strategy was superior to COR for all-cause mortality, cardiovascular mortality, and repeat revascularization. Additionally, ICR reduced the risk of recurrent MI compared to COR. Overall, the results of this meta-analysis demonstrate that ICR or SCR or MV-PCI is superior to COR, MV-PCI is superior to ICR and SCR, while there was no significant difference between the two complete revascularization strategies ICR and SCR. However, future trials should update existing evidence. Our systematic review and network meta-analysis provides a comprehensive summary of different revascularization modalities, which is critical for developing policies on MI management.
Although our analysis does demonstrate efficacy of complete revascularization (whether performed upfront or staged), there are several limitations in our study that need to be addressed. Firstly, the studies included in our analysis were from different cohort observations, leading to insufficient persuasive power of the results. Secondly, some eligible studies did not provide specific timing of non-culprit vessel intervention, which added difficulty and inaccuracy to our grouping. Thirdly, although revascularization can reduce mortality and morbidity, adjuvant medical therapies were not considered in comparing revascularization strategies; however, effective concurrent antithrombotic regimens and superior imaging may aid in improving outcomes. Fourthly, differences in practices across centers, timing of treatments, revascularization based on lesion severity, methods used to assess stenosis severity, etc., can all lead to inaccuracies in data. These issues need resolution prior to adopting a revascularization strategy. Although we examined whether multivessel PCI may improve clinical outcomes in NSTEMI patients with multivessel CAD, revascularization strategies can be tailored based on patient condition, urgency of PCI: and surgical support availability. However, in our study, judicious selection of studies performed in NSTEMI patients would represent a real-world application.
Our findings support current clinical practice guidelines for non-culprit complete revascularization. Given the quality of present evidence, it is appropriate to incorporate patient characteristics, clinical data, lesion complexity, feasibility of PCI: and procedural safety as part of a decision algorithm. An ongoing randomized trial (Direct Complete Versus Staged Complete Revascularization in Patients Presenting With Acute Coronary Syndromes and Multivessel Disease; NCT03621501) [38] and a large cohort study – the FIRE trial (TCT-33 Complete Versus Culprit-Only Strategy in Older Patients With MI and Multivessel Disease: Results From a Cohort Study Based on 4 International Registries) [39] – may provide more information on optimal timing of non-culprit vessels.
In conclusion, our network meta-analysis indicates that for patients with NSTEMI and MVD, complete revascularization performed upfront during the index procedure as multivessel PCI or staged intervention is more effective compared to culprit-only revascularization for most follow-ups, with planned second hospitalization complete revascularization MV-PCI possibly being the most favorable. However, the current quality of evidence is low. Patient characteristics, clinical data, lesion complexity, feasibility of intervention, and procedural safety should be considered comprehensively before selecting a revascularization strategy. High-quality, multicenter, large sample randomized controlled trials are warranted to clarify the efficacy and safety of different revascularization strategies in NSTEMI patients with MVD.