Introduction
Papillary thyroid microcarcinoma (PTMC) is a subset of papillary thyroid carcinoma (PTC) characterized by a diameter of ≤ 10 mm, accounting for around 30% of cases [1]. Initially, most PTMC cases were discovered incidentally during pathological evaluations of thyroid specimens after surgery for benign thyroid conditions or during autopsy [1]. However, the growing application of ultrasound (US)-guided fine-needle aspiration biopsy (FNA) for diagnosing thyroid nodules has led to an increased prevalence of PTMC [1]. A study indicated that the incidence of non-incidental papillary thyroid microcarcinoma (NIPTMC) among all thyroid malignancies rose from 16.67% in 2008 to 33.75% in 2016, but the prevalence of incidental papillary thyroid microcarcinoma (IPTMC) declined from 20.83% in 2008 to 13.75% in 2016 [2].
At the moment, numerous guidelines advocate for a non-surgical approach to low-risk PTMC, characterized by the absence of substantial extrathyroidal expansion, lymph node metastases, or distant metastasis [3, 4]. They promote active surveillance (AS) rather than immediate surgery (IS) for low-risk PTMC and transitioning to surgical intervention solely in instances of tumor progression [3, 4]. This is based on the exceedingly low mortality rate of PTMC, approximately 0.3%, and the minimal recurrence rate of roughly 1 to 5% [5]. Nonetheless, the acceptance of AS for low-risk PTMC remains very low, particularly in countries such as Argentina, Brazil, and other Latin American nations [6]. In the absence of clinical or radiological indicators as well as biomarkers that can effectively differentiate the small fraction of aggressive PTMC from the predominant indolent tumors, patients face the potential for aggressive tumor behavior and the resultant psychological distress [7].
US-guided radiofrequency ablation (RFA) and other thermal ablation techniques have emerged as effective and safe alternative treatments for patients with low-risk PTMC who decline AS or surgical intervention [8]. RFA is often preferred for certain applications, especially for delicate tissues such as thyroid nodules or in situations where precise control is crucial due to its safety, predictability, and ease of use, with non-inferior efficacy compared to other thermal ablation methods, including microwave ablation (MWA) [8]. A meta- analysis of 15 studies indicated that through the use of RFA, the full disappearance rate of PTMC at the completion of follow-up was 79%, with a tumor progression rate of only 1.5% and a lymph node metastasis rate of 0.2% [9]. A recent meta-analysis encompassing 8 studies with a follow-up period beyond 3 years indicated that the overall volume reduction ratio (VRR) following RFA reached 99.81% by the end of the follow-up, with a local recurrence rate of only 3.2% [10]. Unfortunately, the evidence comparing RFA with definitive treatment, specifically surgery, for PTMC patients remains ambiguous and inconsistent. This study intends to evaluate the efficacy and safety of RFA in comparison to surgical interventions (lobectomy or total thyroidectomy) for individuals with PTMC.
Material and methods
Eligibility criteria
This review was registered with PROSPERO to provide consistent methods and enhance evidence synthesis (CRD42025639370). Only randomized controlled trials (RCTs) and observational studies (prospective or retrospective) were considered for this review. These studies were chosen according to a predefined PICO framework to guarantee a standardized evaluation of population, intervention, comparison, and outcomes. The inclusion criteria were as follows:
population = adult individuals (aged 18 years or older) who have been diagnosed with a PTMC with the maximum diameter of 10 mm or less;
intervention = received US-guided RFA as the intervention of their PTMC;
control = received surgical procedures in the form of thyroid lobectomy (TL) or total thyroidectomy (TT) for their PTMC;
outcome = have data on the:
efficacy outcomes = procedure time, blood loss, length of hospitalization, tumor progression rate, lymph node metastasis, recurrence rate, thyroid cancer-specific quality of life (THYCA-QoL), and total cost;
safety outcomes = overall complications, transient recurrent laryngeal nerve (RLN) injury, persistent RLN injury, transient hypoparathyroidism, persistent hypoparathyroidism, and hematoma.
Furthermore, we excluded studies that match the following criteria: (1) the study population comprises pediatric patients; (2) the subjects of interest have papillary thyroid carcinoma (PTC) exceeding 10 mm in diameter; (3) the research seeks to evaluate the efficacy of RFA against microwave ablation (MWA) or laser ablation (LA); (4) the studies do not include a comparative group; (5) non-primary investigation; (6) research articles that are not fully accessible or studies that have not been published. This article was composed within its entirety in accordance with the principles established in the PRISMA statement [11].
Search strategy and study selection
An exhaustive search was conducted across multiple databases, including Europe PMC, Cochrane Library, Scopus, and Medline, encompassing all research from inception until January 20th, 2025. The search was limited to English-language publications. The keywords employed for the literature scan were as follows: “(radiofrequency ablation OR RF ablation OR RFA) AND (surgery OR surgical resection OR lobectomy OR thyroidectomy) AND (papillary thyroid microcarcinoma OR PTMC OR papillary microcarcinoma of the thyroid OR micropapillary thyroid carcinoma OR mPTC)”. Supplementary Table 1 provides further details regarding the search methodology used for each database. The records obtained from the database underwent a screening procedure based on their titles and abstracts. The two authors separately performed this screening, and any duplicate papers were eliminated. Any primary investigative paper cited in review articles, systematic reviews, or meta-analyses, which was not identified in the preliminary search, was deemed eligible for inclusion in the study if it met the specified requirements. Subsequently, the two aforementioned authors performed a thorough evaluation of the full-length papers. In the case of disagreement during the article selection process, discussions were initiated to resolve the conflict.
Table 1
Baseline characteristics of included studies
| Study | Design | Radiofrequency ablation (RFA) | Surgery | Follow-up (months) | Study arms | Sample size | Age (years) | Male (%) | Nodule diameter (mm) | Nodule volume (ml) |
|---|---|---|---|---|---|---|---|---|---|---|
| Gong et al. [15] 2024 | PC | US-guided 18-gauge internally cooled electrode with 5 mm or 7 mm active tips (Minimax Medical, China) | Unilateral thyroidectomy with central lymph node dissection (endoscopic or open) | 12 | RFA | 42 | 51.4 ± 10.5 | 38.1% | ≤ 5 | 30.5 ± 10.2 |
| Surgery | 89 | 50.0 ± 14.6 | 43.8% | ≤ 5 | 33.3 ± 15.4 | |||||
| Lan et al. [16] 2020 | PC | US-guided Moving-shot ablation technique Other details not mentioned clearly | Lateral lobectomy: 52.9% Total thyroidectomy: 47.1% | 11.2 ± 13.4 | RFA | 54 | 41.9 ± 10.2 | 22.2% | ≤ 10 | NR |
| Surgery | 34 | 42.4 ± 9.8 | 8.8% | ≤ 10 | NR | |||||
| Song et al. [17] 2021 | RC | US-guided Mobile shooting technique Celon AG RFA System (Olympus, Tokyo, Japan) and 18-gauge disposable Bipolar RF Electrode with 9 mm active tip | Total thyroidectomy with central lymph node dissection | 28.3 ± 9.6 | RFA | 115 | 44.9 ± 10.4 | 15.6% | 6.5 ± 1.9 | 181.6 ± 156.5 |
| Surgery | 103 | 45.4 ± 9.9 | 18.4% | 6.9 ± 1.6 | 198.2 ± 118.8 | |||||
| Yan et al. [18] 2021 | RC + PSM | US-guided Moving-shot and hydrodissection technique Bipolar RFA generator (CelonLabPOWER; Olympus Surgical Technologies Europe) and 18-gauge bipolar RF applicator with 9 mm active tip | Thyroid lobectomy without prophylactic ipsilateral cervical lymph node dissection | 48.3 ± 23.2 | RFA | 332 | 44.1 ± 9.5 | 24.7% | 5.7 ± 2.2 | 91.6 ± 107.5 |
| Surgery | 332 | 43.8 ± 9.5 | 25.3% | 5.5 ± 2.2 | 86.4 ± 106.8 | |||||
| Yan et al. [19] 2023 | RC | US-guided Moving-shot and hydrodissection technique Bipolar RFA generator (CelonLabPOWER, Olympus Surgical Technologies Europe) and an 18-gauge bipolar RF applicator with 9 mm active tip | Thyroid lobectomy with prophylactic central neck dissection | 72.8 ± 15.3 | RFA | 44 | 44.3 ± 10.2 | 22.7% | 5.5 ± 2.3 | 83.2 ± 111.3 |
| Surgery | 53 | 41.6 ± 10.1 | 34.0% | 6.0 ± 2.4 | 111.0 ± 150.5 | |||||
| Zhang et al. [20] 2021 | RC + PSM | US-guided RFA generator (VIVA; STARmed, Goyang, Korea) with 18-gauge monopolar internally cooled electrode | Open thyroid lobectomy or total thyroidectomy | 6.2 ± 4.8 | RFA | 133 | 45.7 ± 9.8 | 51.0% | 5.3 ± 1.7 | 58.2 ± 52.6 |
| Surgery | 133 | 45.6 ± 10.8 | 49.0% | 5.3 ± 1.7 | 61.9 ± 54.1 | |||||
| Zhang et al. [21] 2020 | RC | US-guided Bipolar RFA generator (CelonLabPOWER; Olympus Surgical Technologies Europe, Hamburg, Germany) and 18-gauge bipolar RF applicator with 9 mm active tip | Thyroid lobectomy with central lymph node dissection (CLD): 48.7% Thyroid lobectomy without CLD: 23.8% Total thyroidectomy with CLD: 17.5% Total thyroidectomy without CLD: 10% | 63.9 ± 3.2 | RFA | 94 | 45.4 ± 10.8 | 25.5% | 6.1 ± 2.5 | 175.9 ± 228.3 |
| Surgery | 80 | 44.1 ± 9.6 | 25.0% | 6.0 ± 1.5 | 132.7 ± 94.1 |
Data extraction
Data were extracted into a uniform format, incorporating study information (e.g., study ID, year, design, sample size), details regarding the method and instrument used for RFA, the type of surgery performed, participant demographics (age, sex, nodule diameter and volume), and follow-up length. Additionally, outcomes measured in each study were recorded. The data collection process was performed separately by the two authors, with any discrepancies resolved through discussion.
We divided the outcomes in our study into efficacy and safety outcomes. Procedure time was defined as the duration between the onset of local anesthesia and completion of ablation in the RFA/LA group, and the duration between skin incision and wound closure in the surgery group. Blood loss was defined as the amount of blood in milliliters (ml) lost during the ablation procedure or surgery. Length of hospitalization was considered as the time from completion of thermal ablation or surgery to discharge. Tumor progression rate was defined by any of the following: (a) persistently detected lesion at the ablated tumor confirmed by biopsy; (b) new recurrent PTMC that was separated from the treated tumor confirmed by biopsy; or (c) cervical lymph node metastasis confirmed by biopsy. Lymph node metastasis denotes the dissemination of cancer cells from the primary tumor in the thyroid to adjacent lymph nodes, especially within the cervical region. Recurrence rate was defined as the identification of a newly malignant lesion in the thyroid bed, contralateral lobe, or metastatic lymph nodes occurring more than six months after the initial ablation or surgery, confirmed as malignant through cytology and surgical excision. THYCA-QoL is a scoring system issued by the European Organization for Research and Treatment of Cancer (EORTC) with the purpose of recording and analyzing seven symptomatic problems (neuro-muscular, voice, attention, sympathetic nerve system, oral cavity/larynx, psychology, and sensation) and six events (scar, coldness, stinging hands/feet, weight gain, headache, and sexual desire). The costs related to RFA mainly included pre-operative examinations, the procedure itself, local anesthesia, and the radiofrequency probe. The costs related to surgery covered pre-operative examinations, the surgical procedure (including vital sign monitoring and consumable medical material), general anesthesia, bed costs, nursing charges, and drugs.
Overall complications comprised the total number of complications experienced by the patients during and after the ablation procedure or surgery. Transient RLN injury was defined as impaired movement of one or both vocal cords on laryngoscopy that recovered within 6 months, while permanent RLN injury was defined as an injury that did not recover within six months. Hypoparathyroidism was defined as a parathyroid hormone level < 15 pg/ml 24 hours postoperatively, and permanent hypoparathyroidism was defined as no recovery within 6 months.
Risk of bias assessment
Two independent assessors used standardized assessment tools to evaluate potential bias in each study. To examine potential bias in observational research, we employed the Newcastle Ottawa Scale (NOS), which has three evaluation criteria: purposeful participant selection, comparability across participant groups, and precision of outcome measurements [12]. Research studies that received a score of 7 or higher were classified as high-quality research according to this evaluation method [12].
Statistical analysis
The continuous variable outcomes, excluding total cost, were calculated using the Inverse-Variance algorithm to obtain the mean difference (MD) along with the 95% confidence interval (95% CI). The total cost outcomes were aggregated into standardized mean differences (SMD) owing to variations in the currencies used throughout the included studies. The outcomes of the dichotomous variable were calculated using the Mantel-Haenszel approach to provide the odds ratio (OR) and its associated 95% CI. In the event that zero events occurred in both intervention groups, continuity correction was used by adding 0.5 to each of the four cells. The diversity of participant characteristics, the operator’s learning curve in performing RFA, and the length of follow-up required the acknowledgment of considerable heterogeneity. Random-effects models were used to overcome this issue. The I2 (inconsistency) statistic was used to measure heterogeneity among studies, with values greater than 50% indicating substantial heterogeneity [13]. The data, initially presented as medians with interquartile ranges or medians with minimum-to-maximum ranges, were converted into means and standard deviations for the purpose of meta-analysis pooling. The conversion was executed with the formula devised by Wan et al. [14]. The present study employed a restricted-maximum likelihood random effects approach to conduct a meta-regression analysis for outcomes exhibiting substantial heterogeneity. This analysis sought to evaluate the influence of study-level factors (study design, sample size, follow-up time, age, percentage of male participants, initial nodule diameter, initial nodule volume) on the outcomes of interest and their contribution to heterogeneity. If the number of studies incorporated in the meta- analysis surpassed 10, a funnel plot was used to assess the existence of publication bias. All analyses in this study were performed using Review Manager 5.4 software and Comprehensive Meta-Analysis version 4.
Results
Study selection and characteristics
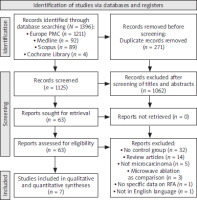
A total of 1,396 publications were obtained from the four databases used for the literature search. Following the removal of all duplicate entries and the assessment of publications based on their titles and abstracts, 1,333 articles were classified as irrelevant and eliminated from further examination. This culminated in the selection of 63 papers for additional analysis. Of the 63 papers evaluated in their entirety, 56 did not meet our established eligibility requirements, and these papers were subsequently eliminated from our study due to the following particular criteria: 32 ar-ti-cles lacked a comparison group, 14 were classified as review articles, 5 assessed papillary thyroid carcinoma with a diameter exceeding 10 mm (thus not qualifying as PTMC), 3 employed MWA as the comparison group, 1 article lacked specific outcome data on RFA, and 1 article was not published in English. In total, 7 papers [15–21] were identified that met the established inclusion criteria and thus selected for final analysis, as depicted in Figure 1. Among the 7 papers examined, 5 were retrospective studies, whereas just 2 were prospective studies. All RFA procedures were carried out under ultrasound guidance, with the moving-shot technique being the predominant method used in the studies reviewed. The most frequent surgical procedures for treating PTMC among the included studies was thyroid lobectomy, with or without central lymph node dissection (CLD). The follow-up period lasted between 6.2 and 72.8 months. Further details regarding the participants’ characteristics and the techniques used for RFA are presented in Table 1.
Quality of study assessment
The evaluation of bias risk in the incorporated studies was performed using the NOS tool. All included observational studies were classified as having “good” quality, with ratings between 7 and 8 (Table 2).
Table 2
Newcastle-Ottawa quality assessment of observational studies
| First author, year | Study design | Selectiona | Comparabilityb | Outcomec | Total score | Result |
|---|---|---|---|---|---|---|
| Gong et al. [15] 2024 | Cohort | *** | ** | *** | 8 | Good |
| Lan et al. [16] 2020 | Cohort | *** | ** | ** | 7 | Good |
| Song et al. [17] 2021 | Cohort | *** | ** | ** | 7 | Good |
| Yan et al. [18] 2021 | Cohort | *** | ** | *** | 8 | Good |
| Yan et al. [19] 2023 | Cohort | *** | ** | *** | 8 | Good |
| Zhang et al. [20] 2021 | Cohort | *** | ** | ** | 7 | Good |
| Zhang et al. [21] 2020 | Cohort | *** | ** | *** | 8 | Good |
Efficacy outcomes
Procedure time
The results of a meta-analysis encompassing 6 studies (n = 1,550) demonstrate that the application of RFA correlated with reduced procedure time in comparison to surgical intervention for the management of PTMC (MD –62.69 min; 95% CI: –78.33, –47.05, p < 0.00001, I2 = 99%, random-effects model) (Figure 2A, Table 3).
Figure 2
Forest plot comparing procedure time (in minutes) (A), blood loss (in milliliters) (B), length of hospitalization (in days) (C), and tumor progression rate (D) between radiofrequency ablation (RFA) and surgery for management of papillary thyroid microcarcinoma (PTMC)
Table 3
Summary of meta-analysis results for each outcome of interest
Blood loss
The findings of a meta-analysis involving 4 studies (n = 1,153) indicate that the use of RFA is associated with less blood loss relative to surgical intervention for the treatment of PTMC (MD –24.13 ml; 95% CI: –30.02, –18.23, p < 0.00001, I2 = 96%, random-effects model) (Figure 2B, Table 3).
Length of hospitalization
Six studies (n = 1,550) reported the length of hospitalization outcome. The comprehensive meta-analysis of these studies indicated that application of RFA resulted in shorter hospitalization compared to surgery in patients with PTMC (MD –6.70 days; 95% CI: –8.85, –4.55, p < 0.00001, I2 = 99%, random-effects model) (Table 2C, Table 3).
Tumor progression rate
A meta-analysis of 6 studies (n = 1,550) revealed that the tumor progression rate was not significantly different between RFA and surgical intervention for the management of PTMC (OR 1.10; 95% CI: 0.52–2.34, p = 0.80, I2 = 0%, random-effects model) (Figure 2D, Table 3).
Lymph node metastasis
A meta-analysis of 6 studies (n = 1,550) showed that the incidence of lymph node metastasis did not differ significantly between RFA and surgical intervention for the treatment of PTMC (OR 0.52; 95% CI: 0.17–1.57, p = 0.24, I2 = 0%, random-effects model) (Supplementary Figure 1, Table 3).
Recurrence rate
There were 6 studies (n = 1,550) that reported the recurrence rate outcome. Pooled analysis from these studies revealed no significant difference in the recurrence rate between RFA and surgery as management for PTMC (OR 1.51; 95% CI: 0.59–3.85, p = 0.38, I2 = 0%, random- effects model) (Supplementary Figure 2, Table 3).
THYCA-QoL
Four studies (n = 611) documented the THYCA-QoL outcome. The extensive meta-analysis of these studies revealed that the THYCA-QoL did not exhibit significant differences between RFA and surgical intervention in the management of PTMC (MD –2.04; 95% CI: –4.73, 0.65, p = 0.14, I2 = 98%, random-effects model) (Supplementary Figure 3, Table 3).
Total cost
Five studies (n = 1,419) documented the total cost outcome. The extensive meta-analysis of these studies revealed a lower total cost among PTMC patients who received RFA than those who were given surgical intervention (std. mean difference –2.77; 95% CI: –4.27, –1.27, p = 0.0003, I2 = 99%, random-effects model) (Supplementary Figure 4, Table 3).
Safety outcomes
Overall complications
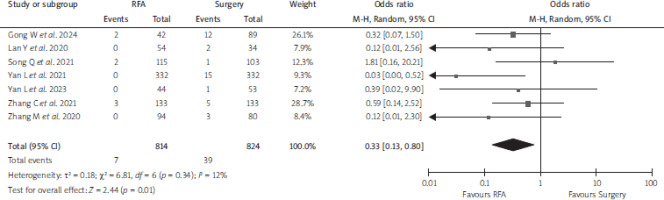
Seven papers (n = 1,638) reported the overall complications outcome. Pooled analysis from these studies showed a lower overall complication rate among PTMC patients who received RFA than those who received surgery (OR 0.33; 95% CI: 0.13–0.80, p = 0.01, I2 = 12%, random- effects model) (Figure 3, Table 3).
Transient RLN injury
A comprehensive meta-analysis including 6 studies (n = 1,541) revealed no statistically significant difference in transient RLN injury between RFA and surgical intervention for PTMC (OR 0.46; 95% CI: 0.14–1.49, p = 0.19, I2 = 32%, random-effects model) (Supplementary Figure 5, Table 3).
Persistent RLN injury
A comprehensive meta-analysis including 7 studies (n = 1,638) revealed no statistically significant difference in persistent RLN injury between RFA and surgical intervention for PTMC (OR 0.47; 95% CI: 0.12–1.75, p = 0.26, I2 = 0%, random-effects model) (Supplementary Figure 6, Table 3).
Transient hypoparathyroidism
A total of 5 papers (n = 1,419) reported the incidence of transient hypoparathyroidism. The results of these 5 studies reveal no statistically significant difference between RFA and surgery for the incidence of transient hypo-parathyroidism (OR 0.45; 95% CI: 0.11–1.87, p = 0.27, I2 = 0%, random-effects model) (Supplementary Figure 7, Table 3).
Persistent hypoparathyroidism
A total of 5 papers (n = 1,419) reported the incidence of persistent hypoparathyroidism. The results of these 5 studies reveal no statistically significant difference between RFA and surgery for the incidence of persistent hypo-parathyroidism (OR 0.72; 95% CI: 0.13–3.83, p = 0.70, I2 = 0%, random-effects model) (Supplementary Figure 8, Table 3).
Hematoma
Overall meta-analysis from 3 studies (n = 494) in PTMC patients showed no significant difference in the hematoma rate between RFA and surgery (OR 0.61; 95% CI: 0.08–4.42, p = 0.63, I2 = 0%, random-effects model) (Supplementary Figure 9, Table 3).
Meta-regression
The findings of the meta-regression analysis are presented in Supplementary Table 2. The regression analysis revealed that the procedure time outcome was not significantly affected by study-level factors, such as study design (p = 0.5597) (Supplementary Figure 10A), sample size (p = 0.3324) (Supplementary Figure 10B), follow-up time (p = 0.1245) (Supplementary Figure 10C), age (p = 0.2161) (Supplementary Figure 10D), percentage of male participants (p = 0.5598) (Supplementary Figure 10E), initial nodule diameter (p = 0.5656) (Supplementary Figure 10F), or initial nodule volume (p = 0.4782) (Supplementary Figure 10G). The substantial heterogeneity in procedure time outcome cannot be explained by these factors.
Our meta-regression analysis also indicated that study-level variables – including follow-up duration (b coefficient: –0.0788; 95% CI: –0.1520, –0.0057; p = 0.0346) (Supplementary Figure 11A), initial nodule diameter (b coefficient: –4.6860; 95% CI: –8.0128, –1.3592; p = 0.0058) (Supplementary Figure 11B), and initial nodule volume (b coefficient: –0.0467; 95% CI: –0.0665, –0.0269; p < 0.0001) (Supplementary Figure 11C) – were significantly associated with length of hospitalization and contributed to the heterogeneity found in this outcome of interest. Meanwhile, other factors – including study design (p = 0.1565) (Supplementary Figure 11D), sample size (p = 0.9940) (Supplementary Figure 11E), age (p = 0.1055) (Supplementary Figure 11F), and percentage of male participants (p = 0.0788) (Supplementary Figure 11G) – were not statistically significantly associated with this particular outcome.
Furthermore, the findings of the meta-regression ana-lysis showed that age (b coefficient: –4.6570; 95% CI: –8.2646, –1.0493; p = 0.0114) (Supplementary Figure 12A), percentage of male participants (b coefficient: 0.8731; 95% CI: 0.1094, 1.6368; p = 0.0251) (Supplementary Figure 12B), initial nodule diameter (b coefficient: –10.4980; 95% CI: –12.7885, –8.2074; p < 0.0001) (Supplementary Figure 12C), and initial nodule volume (b coefficient: –0.1015; 95% CI: –0.1235, –0.0795; p < 0.0001) (Supplementary Figure 12D) were statistically significantly associated with the blood loss outcome and contributed to the substantial heterogeneity found in this particular outcome. In contrast, other study-level factors such as sample size (p = 0.5296) (Supplementary Figure 12E) and follow-up time (p = 0.2420) (Supplementary Figure 12F) did not show a significant association with the blood loss outcome.
Additionally, our regression analysis indicated that the total cost outcome, including the notable heterogeneity observed, was only significantly associated with follow-up time (beta coefficient: 0.3284; 95% CI: 0.0424, 0.6143; p = 0.0244) (Supplementary Figure 13A). Potential associations of other study-level factors with the total cost outcome were also examined, but no significant associations were found: sample size (p = 0.9239) (Supplementary Figure 13B), age (p = 0.1882) (Supplementary Figure 13C), percentage of male participants (p = 0.8289) (Supplementary Figure 13D), initial nodule diameter (p = 0.2094) (Supplementary Figure 13E), and initial nodule volume (p = 0.1987) (Supplementary Figure 13F).
Finally, the results of the regression analysis indicated that several study-level factors were significantly associated with the THYCA-QoL outcome. These factors include study design (β coefficient: 5.8122; 95% CI: 0.8273–10.7971; p = 0.0223) (Supplementary Figure 14A), sample size (β coefficient: 0.0715; 95% CI: 0.0614–0.0815; p < 0.0001) (Supplementary Figure 14B), and percentage of male participants (β coefficient: –0.3345; 95% CI: –0.4178 to –0.2511; p < 0.0001) (Supplementary Figure 14C). Nevertheless, the regression analysis also revealed that the THYCA-QoL outcome was not significantly associated with follow- up time (p = 0.5110) (Supplementary Figure 14D) or age (p = 0.3327) (Supplementary Figure 14E).
Publication bias
Funnel plot analysis was considered to evaluate publication bias. However, this study did not evaluate publi-cation bias due to the insufficient number of included studies (fewer than 10 papers). According to the literature, publication bias is not considered reliable when fewer than 10 papers are available for analysis [22, 23].
Discussion
The results of our meta-analysis indicated that, regarding efficacy, RFA offers benefits over surgery for patients with PTMC, including reduced procedure duration, less blood loss, shorter hospital stays, and lower overall patient expenses. These benefits demonstrated by RFA are unsurprising, as it is fundamentally a less invasive intervention than surgery, hence reducing the time needed to perform the procedure and expediting patient recovery, which in turn minimizes hospital care costs. This study also successfully addressed previous concerns about RFA regarding potential residual tumor cells after ablation, posing a risk of tumor progression through metastasis or recurrence. Our meta-analysis demonstrated no significant difference in tumor progression rate, lymph node metastasis, or recurrence rate between RFA and surgery for PTMC patients. Moreover, regarding the safety profile, RFA demonstrated a reduced overall number of complications compared to surgery; however, we did not observe any differences in transient and persistent RLN damage, transient and persistent hypoparathyroidism, or hematoma occurrence.
Subsequent regression analysis in the current review revealed that study-level variables – including study design, sample size, follow-up duration, age, sex, initial nodule diameter, and initial nodule volume – were significantly associated with the overall effect size, and considerable heterogeneity was observed in several outcomes, such as blood loss, length of hospitalization, THYCA-QoL, and total cost. Our meta-regression did not identify any association of the aforementioned study-level covariates with the procedure time outcome. We hypothesize that the significant variability observed in procedure time outcome is affected by the disparities in the learning curve and the expertise of operators conducting RFA and surgery.
According to our knowledge, this is the first systematic review and meta-analysis that thoroughly compares the application of RFA and surgery as therapeutic interventions for patients with PTMC. The prior meta-analysis conducted by Sun et al. [24] in 2022 concentrated on PTC in its entirety rather than specifically on PTMC. Two of the 6 studies considered – those by He et al. [25] and Xiao et al. [26] – involved patients with PTC, defined as tumors with a diameter exceeding 10 mm. In our opinion, it is inappropriate to combine tumors with a diameter of ≤ 10 mm with those exceeding 10 mm, as these two categories exhibit distinct characteristics and require different therapeutic strategies. Research indicates that extrathyroidal extension and lymph node metastases are more prevalent in PTC with a diameter exceeding 10 mm than those with a diameter of 10 mm or less [27, 28]. The American Thyroid Association (ATA) guidelines do not recommend the biopsy of thyroid nodules of 10 mm or below, provided there is no sign of extrathyroidal extension or metastasis, even if ultrasonography reveals highly concerning characteristics [29]. Conversely, the same guidelines strongly advocate using US-guided FNA in thyroid nodules exceeding 10 mm in diameter that have an intermediate to high suspicion ultrasonography pattern [29]. Furthermore, thyroid lobectomy alone is adequate as a definitive treatment for PTMC (diameter ≤ 10 mm) in patients who have not undergone prior head or neck radiation, do not have familial thyroid carcinoma, and lack unfavorable conditions such as gross extrathyroidal extension, central-compartment neck lymph node metastasis, or lateral-compartment neck lymph node metastasis [29]. In contrast, the definitive treatment for PTC above 10 mm is near-total or complete thyroidectomy [29]. Consequently, for our present study, we opted to concentrate on PTMCs and omitted studies concerning PTCs above 10 mm in diameter.
Furthermore, upon closer examination, the study by Xiao et al. [26], which was part of the earlier meta-analysis by Sun et al. [24], did not compare RFA with surgery for PTC; it only assessed the effects of US-guided RFA on T1aN0M0 PTC and T1bN0M0 PTC. Consequently, the research conducted by Xiao et al. [26] fails to satisfy the inclusion criteria and should be excluded from the analysis. Our current evaluation encompasses a total of 7 studies that explicitly compare RFA with surgery for PTMC patients.
The prior meta-analysis conducted by Sun et al. [24] analyzed only two outcome types – tumor progression rate and total complications – so we are unable to obtain a clear picture of the comparative effectiveness and safety of RFA and surgery for PTMC patients. Conversely, the present study examined 14 outcomes categorized into 8 efficacy parameters, including total cost, and 6 safety parameters, yielding more comprehensive evidence. Additionally, this review incorporated meta-regression analysis to investigate the associations of study-level variables with the outcomes of interest and their contribution to the observed heterogeneity.
This study has some limitations. First, the review included a limited number of studies, with the majority exhibiting sample sizes under 100 participants. Hence, the conclusions drawn from this study have limited generalizability. Secondly, the data generated in our meta-analysis predominantly originate from retrospective studies, which are prone to the effects of information and selection bias, together with potential confounding variables. Therefore, it is essential to be cautious when interpreting the findings of our study, given this limitation. Moreover, a significant degree of hete-rogeneity was noted in the several outcomes of interest within this study, attributable to differences in baseline characteristics among participants, variations in the techniques and instruments applied in RFA, and disparities in the expertise of the medical professionals performing the ablation and surgical procedures. Also, for certain outcomes exhibiting a double-zero event in both groups, we implemented a continuity correction by adding a value of 0.5 to each cell. This may not be the best method to handle double-zero events and could still introduce bias into the results; therefore, the findings from these outcomes should be interpreted cautiously. We contend that the findings from our comprehensive investigation and meta-analysis can offer valuable insights for enhancing the therapy of PTMC.
Conclusions
The results of our in-depth systematic review and meta- analysis showed that RFA was more effective than surgery in terms of shorter procedure times, less blood loss, faster hospital discharge, and lower overall costs for PTMC patients. RFA also demonstrated similar efficacy to surgery regarding tumor progression rate, lymph node metastasis, and recurrence rate. Regarding safety, RFA demonstrated a lower incidence of overall complications compared to surgery, with comparable rates of transient and persistent RLN damage, transient and persistent hypoparathyroidism, and hematoma occurrence. US-guided RFA may be regarded as a feasible option for the management of PTMC, especially for patients who refuse AS or are ineligible for surgical procedures. Furthermore, it is highly advisable to undertake meticulously planned RCTs with significant sample numbers and extended follow-up periods to validate the findings of our study.