Summary
Coronary calcifications in the left main disease are associated with the high complexity of percutaneous coronary intervention, increased rate of periprocedural complications, and poorer outcomes. The presence of undilatable lesions, resistance to the high-pressure non-compliant balloon inflation is a rare, but highly demanding clinical scenario. Our data suggests that the use of shockwave intravascular lithotripsy (S-IVL) could be safe and allow for adequate lesion preparation before stent implantation. Despite encouraging results, future large-number studies with a long-term observation are necessary to evaluate the safety and efficacy of S-IVL in left main stenosis.
Introduction
Traditionally, a surgical approach has been dedicated for coronary artery disease (CAD) with significant left main (LM) stenosis. However, current European guidelines for percutaneous coronary intervention (PCI) [1] support PCI in LM disease when characterized by a low or intermediate Syntax Score. The prevalence of LM stenosis is relatively low in patients undergoing routine angiography. However, it is more common in the acute coronary syndrome setting. Furthermore, due to the aging process and numerous comorbidities, the number of patients with a high risk of surgical intervention or disapproving of such therapy is instantly growing [2]. Since left main disease is related to the large myocardial ischemic territory, the high-risk patients are frequently qualified for rescue percutaneous procedures.
Growing evidence indicates improvement in the short- and long-term outcomes of LM-related PCI [3, 4]. However, the efficacy of PCI in highly calcified lesions is poorer [5]. Calcifications complicate all steps of the standard PCI procedure starting from lesion crossing (with a guidewire, balloon, or stent) to inadequate stent expansion. The crucial point to avoid these unfavorable clinical events is aggressive plaque modification before coronary stent implantation. Numerous strategies and dedicated devices aiming at appropriate lesion preparation of calcified plaques have been implemented in clinical practice [6].
Mentioned devices can be assigned to two main categories. First, balloon-dependent (non-compliant (NC), ultrahigh-pressure balloon (OPN), or cutting/scoring catheters) devices focus on the exertion of internal pressure on the lesion. Second, atherectomy-dependent (rotational, laser, and orbital) devices focus on defragmentation and removal of atherosclerotic plaque. Although the success rate of these strategies is high and reaches over 90.0% [7], still all of them have some limitations.
Therefore, clinical trials focused on new methods of calcified plaque modification are eagerly undertaken. Recently, a novel device has been proposed – Shockwave C2 Intravascular Lithotripsy (S-IVL) (Shockwave Medical Inc, Santa Clara, United States), which is a balloon-based coronary system for IVL transforming electrical energy into mechanical energy, leading to profound defragmentation of calcium deposits. The evidence for the efficacy and safety of S-IVL is mainly provided by studies in the field of peripheral arterial atherosclerosis and a few real-life registries [8]. Furthermore, patients with LM disease are significantly underrepresented or had been excluded from the studies.
Aim
Therefore, we designed this study to evaluate the efficacy and safety of S-IVL in the management of calcified unprotected left main stenosis.
Material and methods
In this retrospective observational registry, we included patients from two cooperative cardiac centers. All subjects had moderate or severely calcified LM stenosis with a clinical indication for PCI (heart team judgment, ongoing ischemia or patient’s volition). Due to initial unsuccessful attempts of plaque modification by other devices (including the NC balloon catheters or rotational devices) the S-IVL was applied. We performed all PCI procedures between May 2019 and May 2021.
Unsuccessful plaque modification was defined as significant under-expansion (20% of balloon diameter at least 18 atm inflation) on a NC balloon (sized 1 : 1 to the reference vessel diameter) despite initial aggressive plaque modification maneuvers.
Baseline and final coronary angiograms were recorded and analyzed. Post-procedural evaluation (quantitative coronary angiography) was performed using angiographic software – Artis ZEE (Siemens Healthcare, Erlangen, Germany). Measurements were performed using the same single worst-view projection. The catheter tip was used for initial calibration.
We assessed calcific deposits angiographically as mild (spots), moderate (involving ≤ 50% of the reference lesion diameter), or severe (involving > 50% of the reference lesion diameter). There were no vessel-related exclusion criteria regarding lesion anatomy, length, tortuosity, severity, or prior stent placement.
The study evaluated the safety and efficiency of S-IVL in LM disease. Therefore, the study had two primary endpoints: clinical success and subsequently the safety-related outcomes. Clinical success was defined as effective stent delivery and deployment (with less than 20% in-stent residual stenosis [9, 10]) with preserved Thrombolysis in Myocardial Infarction (TIMI) 3 flow at the end of the procedure.
Safety outcomes were defined as procedural complications (coronary perforation, slow or no reflow, new coronary thrombus, ventricular arrhythmias, vessel closure, inability to deliver the stent or inappropriate expansion) as well as device failure (inability to cross the lesion, malfunction, or rupture). Major adverse cardiac and cerebrovascular events (MACCE) were defined as an myocardial infarct, cerebrovascular events, major bleeding, need for repeated revascularization, or death in accordance with a definition proposed in the fourth universal definition for myocardial infarction [11]. Clinical follow-up was obtained by telephone contacts or personal visits at the 30th day after the index procedure (additional 6 and 12 months follow-up is ongoing and will be reported when completed).
Results
The study population consisted of 16 patients, mainly males (81.3%) with CAD treated with left main S-IVL PCI. The vast majority of patients had a high-risk profile with a high prevalence of risk factors and previous cardiac history. The most frequent indication for PCI was acute coronary syndrome (10 (62.5%)). The mean left ventricular ejection fraction was 49.5 ±18.1%. In 1 case, we performed PCI with the support of a ventricular assist device – Impella (Abiomed, Danvers, Massachusetts). Subsequently, two subjects required periprocedural use of catecholamines.
Most procedures were performed using the 7F 11 (68.7%) and radial access 13 (81.2%). After pre-treatment with high-pressure NC balloon catheter inflation, the S-IVL balloon was delivered into a body of a lesion in all treated subjects. Additionally, in 1 case, rotational atherectomy was performed. The S-IVL balloon was sized (3.4 ±0.4 mm angiographically (balloon/vessel ratio 1 : 1) with an average pulse number of 46.8 ±27.5 per procedure.
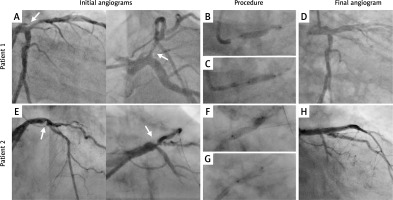
All patients achieved PCI-related clinical success with the average post-PCI stenosis of 6%. There was no coronary perforation, impaired flow, thrombus formation, or ventricular arrhythmias. There were 2 cases of MACCE in the 30-days follow-up period. One lethal in-stent thrombosis occurred during the in-hospital observation period (5 days after a PCI). The second was a death for an unknown reason 14 days after discharge (patient with a high number of comorbidities and low left ventricular ejection fraction (LVEF) (25%) – primarily qualified for scheduled cardioverter-defibrillator (ICD) implantation). Table I contains the basic clinical and procedural characteristics of all cases included in the study. Examples of S-IVL–LM procedures are presented in Figure 1.
Table I
Clinical, procedural, and postprocedural characteristics of the study population (n = 16)
[i] STEMI – ST-elevation myocardial infarction, NSTEMI – no ST-elevation myocardial infarction, LM – left main, LAD – left anterior descending, Cx – circumflex artery, RCA – right coronary artery, LVEF – left ventricular ejection fraction, CTO – chronic total occlusion, IVL – intravascular lithotripsy, DES – drug eluting stent, DEB – drug eluting balloon, OCT – optical coherence tomography, IVUS – intravascular ultrasound, MACCE – major adverse cardiac and cerebrovascular events.
Discussion
Current guidelines [1] strongly recommend revascularization in all patients with ≥ 50% stenosis of the LM. Lesions in this location are often symptomatic due to large myocardial ischemia territory resulting in high prognostic risk. Moreover, calcifications in left main disease are one of the strongest mortality predictors [12]. Notably, the presented registry includes these high-risk patients with clinical indications for PCI and co-occurring initial unfavorable results of lesion preparation with a non-compliant balloon. Nowadays, atherectomy techniques are often used in such cases. Compared to classical balloon-dependent devices, rotational atherectomy [13] and orbital atherectomy [14] are associated with an increased procedural success rate, especially when severe calcifications occur.
Despite the substantial amount of evidence regarding the efficacy of rotational devices, data on the treatment of LM stenosis are scarce. Due to numerous safety concerns, mainly slow flow or perforation with subsequent sudden hemodynamic deterioration, patients with LM disease are often excluded from studies or receive surgical treatment. Data from small observational RA registries [15–17] suggest favorable short-term results, with in-hospital rate of MACCE in the range 5.8–13.4% and in-hospital death from 2.8% to 5.9%. Analogous data regarding orbital atherectomy are limited [18], but they suggest similar efficacy and safety [19].
The armamentarium for optimal lesion preparation in bail-out settings of “undilatable lesion” has recently been enlarged and the preliminary data on S-IVL effects are encouraging [10, 20, 21] This novel device adapted from peripheral interventional techniques [22] seems to be a reasonable alternative to rotational devices. However, convincing data regarding the LM stenosis are missing. Only a few low power studies [23–25] have been conducted so far. Short-term observations in this high-risk group seem to be favorable – the 30-day MACE rate was in the range of 3.2–12.5%. These results are consistent with those observed in our registry. Similar to our results, in all mentioned studies the PCI success rate was 100% and despite high complexity of the lesions, the vast majority of procedures (over 80%) were performed via radial access, which resulted in significant reduction of the access-site complications. Also, none of the typical complications related to rotational devices (slow flow; perforation) appeared in our or other available studies.
Due to the novelty of the Shockwave technology, the present study has some limitations: retrospective nature, lack of a comparator group, and small underpowered sample size. Also, the low prevalence of intravascular imaging and lack of external core lab assessment is an important limitation of the study.
Despite the mentioned limitations, we found some potential advantages of this device in LM stenosis compared to other plaque modification techniques. The S-IVL system is a relatively easy to use balloon-dependent device. Since allowing for use of standard guidewires, it results in a shortened learning curve with subsequent reduction in device failure and complication rates. Moreover, an undeniable advantage of this technology is its ability to be applied in large vessels and its suitability for treatment of previously suboptimal implanted scaffolds [26, 27] and calcified saphenous vein grafts [28]. Moreover, compared to rotational devices, an additional guidewire may be left in site branches during the procedure, which facilitates performing the two-stent-strategy PCI.
On the other hand, some concerns regarding the performance and safety of the S-IVL device cannot be omitted. In tight, severely calcified lesions, the bulky character of a device can preclude system delivery into body lesions. However, recently Rota-lithotripsy – a combination of rotational atherectomy and Shockwave Intravascular Lithotripsy – has been proposed as a bail-out strategy [29–31] for these high-demanding patients and was also applied in our study. Therefore, in most of the cases, the initial NC balloon predilation was sufficient to facilitate the S-IVL passage.
Some data suggest increased platelet activation [32] resulting from the Shockwave therapy. However, the exact effect of S-IVL on thrombogenesis remains unclear.
Conclusions
Coronary calcifications in left main disease are associated with the high complexity of PCI, increased rate of periprocedural complications, and poorer outcomes. The presence of undilatable lesions and resistance to the high-pressure NC balloon inflation is a rare, but highly demanding clinical scenario. Our data suggest that the use of S-IVL could be safe and allow for adequate lesion preparation before stent implantation. Despite encouraging results, future large studies with long-term observation are necessary to evaluate the safety and efficacy of S-IVL in LM stenosis.