Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are acute onset syndromes affecting the lungs directly or indirectly, which develop for several reasons and are characterized by hypoxemia and diffuse lung infiltration. Cases with an oxygenation rate of PaO2/FiO2 ≤ 300 are defined as ALI, and those with an oxygenation rate of PaO2/FiO2 ≤ 200 are defined as ARDS [1]. ALI and ARDS are diseases with a poor prognosis often encountered in intensive care, which begin with moderate pulmonary function disorder and continue progressively and finally result in fatal pulmonary insufficiency [2, 3]. Despite the recent increase in the number of studies conducted on intensive care patients and new therapeutic developments, ALI remains an important clinical problem with high mortality rates [4].
The chemical structure of thymoquinone (TQ) is C10H12O2 and it is a bioactive component obtained from nigella oil. TQ is known to have antihypertensive, antidiabetic, antibacterial, anti-inflammatory, neuroprotective and antiapoptotic effects [5–10]. TQ inhibits cyclooxygenase (COX) and 5-lipooxyenase pathways in arachidonic acid metabolism. It has been shown to inhibit thromboxane B2 and leukotriene B4 formations depending on the dosage [11]. COX-2 is an inducible form of COX and the level has been shown to increase in inflammation [12]. It has been demonstrated that COX-2 causes an increase in the inflammatory immune response in pulmonary damage [13]. The pharmacological inhibition of COX-2 has been determined to show protective activity against ALI by causing a reduction in proinflammatory cytokines and chemokines [14]. The activity of TQ is known in lipopolysaccharide (LPS)-induced acute lung injury. It is considered that it could be effective in ALI/ARDS treatment by ensuring possible COX-2 inhibition.
Aim
The purpose of this study was to research the protective activity of TQ in an LPS-induced ALI model.
Material and methods
Ethics statement
All experimental procedures have been validated with the protocol 88/2013 in accordance with the requirements of the Dokuz Eylul University Animal Care and Ethics Committee.
Materials
TQ was purchased from Sigma-Aldrich Inc. (MO, USA). LPS from Escherichia coli 055:B5 (Sigma-Aldrich, St. Louis, MO, USA) was used to induce ALI.
Experimental design
The study included 28 BALB/C male mice, aged 6–8 weeks, each weighing 20–32 g. The mice were kept at room temperature (21–22°C) and 40–60% relative humidity in a 12-hour light and 12-hour dark environment and fed with standard pellet feed and water. The murine model used by Shirley et al. was used in the study in order to create acute lung injury. A lipopolysaccharide-induced ALI model was created [15].
Anesthesia was ensured through intraperitoneal (ip) 50 mg/kg ketamine and 10 mg/kg xylazine for mice in all the groups. When necessary, maintenance ketamine (at half dose, 25 mg/kg) was repeated according to the reflex responses (painful stimulant to foot via forceps – pedal reflex, palpebral and corneal reflexes) in order to maintain a stable anesthesia depth.
The mice were divided into 4 groups as group I (n = 7) to which intra-tracheal serum and intraperitoneal serum were administered physiologically, group II (n = 7) to which intraperitoneal 3 mg/kg TQ and intra-tracheal serum were administered physiologically, group III (n = 7) to which intra-tracheal 5 mg/kg LPS and intraperitoneal serum were administered physiologically, and group IV (n = 7) to which intra-tracheal 5 mg/kg LPS and intraperitoneal 3 mg/kg TQ were administered [7].
The intraperitoneal injection of TQ was applied 1 hour before the lipopolysaccharide application. Tissue samples were taken 6 hours after the lipopolysaccharide application [16]. The mice were sacrificed and the lungs were extracted. The tissue samples were fixed for 24 hours in 4% neutral buffered formalin. Sections of 4 µm thickness were taken following the routine tissue follow-up procedure and stained with hematoxylin-eosin.
Histological analysis
The samples analyzed via light microscope were evaluated in terms of intra-arterial thrombus, neutrophils, intra-alveolar hemorrhage, alveolar destruction, interstitial edema and hyaline membrane. The samples were graded as (–) if they had no symptoms and the samples showing symptoms were graded as mild (+), moderate (++) and severe (+++) according to the condition. The evaluation was applied with the lung injury semi-quantitative scoring system suggested by Shields [17]. In this semi-quantitative scoring system, 0 indicated no change, 1+ focal mild changes, 2+ multifocal mild changes, and 3+ diffuse severe changes.
Statistical analysis
The statistical analysis of the data was performed with the SPSS 15.0 program. Values were stated as mean, median, standard deviation and minimum and maximum. The Kruskal-Wallis test was performed to test the significance of the difference between the mean and 4 groups with no normal distribution. The Mann-Whitney U test was used for pairwise comparison of the groups and all data were shown as mean ± standard deviation (mean ± SD). A value of p < 0.05 was accepted as statistically significant.
Results
The lung tissue sections of 28 mice were evaluated in terms of intra-arterial thrombus, neutrophil migration, intra-alveolar hemorrhage, alveolar destruction, interstitial edema and hyaline membrane symptoms.
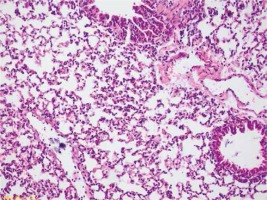
No intra-arterial thrombus, interstitial edema or hyaline membrane formation was determined in group I. It complied with normal lung tissue (Figure 1). Neutrophil migrations were determined in all cases in Group II and mild intra-alveolar hemorrhages were observed in almost half of the cases. The alveolar destruction was mild in almost all cases (Figure 2). Increases were seen in the degrees of neutrophil migration, intra-alveolar hemorrhage and alveolar destruction in group III compared to group I and group II (Figure 3). Decreases were seen in the degrees of neutrophil migration, intra-alveolar hemorrhage and alveolar destruction in group IV compared to group III (Figure 4). No hyaline membrane formation or intra-arterial thrombus was determined in any case. When all the study groups were compared, significant differences were found between the groups in terms of the degrees of neutrophil migration (p = 0.042), intra-alveolar hemorrhage (p = 0.004) and alveolar destruction (p = 0.0006) (Tables I, II).
Figure 2
Group II slight injury in lung alveolar tissue (arrowhead), tissue increase in peribronchial area (star), focal neutrophilic infiltration symptoms (HE, 40×)
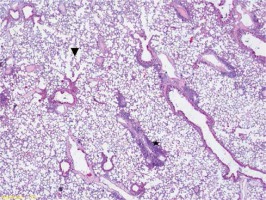
Figure 3
Group III very intense inflammation involving neutrophils (star), intra-alveolar hemorrhage (arrow) alveolar destruction and edema symptoms (arrowhead) (HE, 200×)
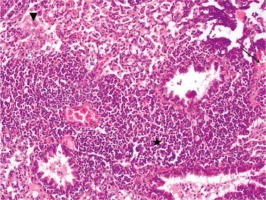
Figure 4
Group IV focal alveolar hemorrhage (arrow), focal slight edema symptoms (arrowhead) (HE, 100×)
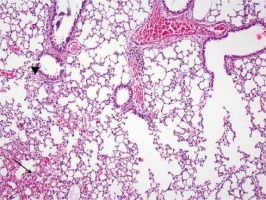
Table I
Pathology results obtained in all study groups
Table II
Gradation of histopathological examinations
Discussion
This experimental study analyzed the protective activity of TQ in LPS-induced lung injury. Apparent increases were determined in the degrees of neutrophil migration, intra-alveolar hemorrhage and alveolar destruction in the LPS group compared to the other groups. The lung histopathology in the TQ treatment group showed similarities with the control group and the groups in which only TQ was administered. This is the first experimental study which shows the protective activity of TQ in LPS-induced lung injury. Although at a lesser degree in the present study, alveolar destruction, neutrophil migration and intra-alveolar hemorrhage were seen in Groups I and II and this was considered to be associated with the trauma created by the intratracheal injection.
Deterioration of the alveolar capillary membrane integrity, excessive transepithelial neutrophil migration and the release of pro-inflammatory, cytotoxic mediators, anti-inflammatory cytokines, proteases and oxidants are the cellular characteristics of acute lung injury [18, 19]. Active neutrophils release oxidant and proteolytic enzymes and these damage the basal membrane and epithelium cells [20, 21]. Neutrophils settle in the lung interstitium in the proliferative phase and release mediators causing chemotaxis [22]. LPS, which is a structural component of the outer membrane of Gram-negative bacteria, acts potently to form ALI. Neutrophil-dependent ALI, which induces regional recruitment and activation of neutrophils, extrication of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 and the production of reactive oxygen and nitrogen species, is also known as LPS-induced ALI [16].
TQ is a bioactive monomer derived from black seed (Nigella sativa) oil, which has been reported to exhibit many pharmacological effects, including immunomodulatory, anticancer, antioxidant, and anti-inflammatory effects [23]. TQ inhibits the cyclooxygenase and 5-lipooxygenase pathways in the arachidonate metabolism. It has been shown to affect the formation of thromboxane B2 and leukotriene B4 by inhibition in a dose-related manner [7]. COX-2 is an inducible isoform of COX and it has been found at increasing levels during inflammation [12]. COX-2 has been shown to cause an increase in the inflammatory immune response in lung injury [13]. It has been determined that the pharmacological inhibition of COX-2 shows protective activity against ALI/ARDS by causing a decrease in proinflammatory cytokines and chemokines [14]. From the results of this study, TQ was considered to show protective activity against ALI through COX inhibition and antioxidant activities.
To the best of our knowledge, there has been no previous study in the literature which has analyzed TQ activity in LPS-induced acute lung injury. There is only one study, by Isık et al., which analyzed TQ activity in acute lung injury created in rats exposed to human intratracheal gastric juice [24]. In contrast to the present study, 6 mg/kg of TQ was administered to the rats and the model was created with human intratracheal gastric juice. Recoveries both in the oxygenation and lung histopathology were observed in the rats administered TQ in that study. However, no decrease in CO2 levels was observed, which was explained by insufficient ventilation of the group of rats administered TQ. There are some limitations associated with the present study. Electron microscopic examination could not be made and oxidant and antioxidant levels could not be observed in this study because of a lack of technical facilities.
The results of the present study showed the protective activity of TQ in LPS-induced lung injury. It is considered that this effect was achieved through antioxidant and COX-2 inhibition. With the inhibition of COX-2, leukotriene, thromboxane and prostaglandin formations are inhibited. Decreases are observed in neutrophil chemotaxis, vasoconstriction, bronchospasm and vascular permeability with the decrease of leukotriene formation. There will be a decrease in inflammatory cell infiltration with the decrease of neutrophil chemotaxis. However, the serine proteases, oxidants and matrix metalloproteinases released from neutrophils show a decrease, so the formation of tissue damage is prevented [11].