The success rate of flexible bronchoscopic intubation via a supraglottic airway device (SAD) ranges from 76% to 100% [1]. This technique, which can be applied using direct or indirect methods, is recommended by difficult airway management guidelines [2, 3]. The main advantage of the direct method is that it contains fewer steps. An Aintree Intubation Catheter™ (Cook India Medical Devices, Tamilnadu, India) is often used as an indirect method. It was proposed in 2011 in the Difficult Airway Society guideline, and the steps were described [4]. The laryngeal mask airway (LMA) Supreme™ (LMA Supreme, Teleflex, Morrisville, NC, USA) is not recommended according to this manual.
There are different types of SADs on the market and intubation through these devices is a valuable difficult airway management strategy [5]. However, both pharyngeal positions and oropharyngeal cuff leak pressures may differ [6]. Studies on the ideal device for fibreoptic intubation through an SAD are limited, and results are contradictory. As most comparative studies have been performed without the Aintree catheter, there is a lack of data in this field.
Highlights of the i-gel® (Intersurgical Ltd, Berkshire, UK) include a non-inflatable cuff, bite-block, and gastric drainage channel [7]. The wide oval structure of the i-gel provides buccal stabilization. Thus, the risk of axial rotation and malposition is reduced. These features make the i-gel an ideal conduit for fibreoptic tracheal intubation. Michalek et al. [8] compared the i-gel with the intubating laryngeal mask airway and CTrach™ laryngeal mask (The Laryngeal Mask Company, Singapore), and in this study, the i-gel was found superior in terms of both insertion and tracheal intubation times. However, in another randomised study, the i-gel and the LMA Protector™ (Teleflex Medical, Co. Westmeath, Ireland) were compared without using an Aintree catheter, and no significant difference was found between the two devices [9]. Complications have been reported with both SADs. In a multicentric observational study, the most common complication for the i-gel was reported to be blood-stained airway devices followed by laryngospasm [10]. However, unilateral hypoglossal nerve palsy has been reported with the use of the LMA Protector [11]. In this study, our primary aim was to compare the efficiency of the LMA Protector and the i-gel as a conduit for Aintree catheter-guided fibreoptic tracheal intubation.
METHODS
The study was approved by the Ethical Review Board of Kocaeli Medical School (KIA 2018/77). Written informed consent was obtained from all subjects. The study was designed prospectively and registered before patient enrolment at www.ClinicalTrials.gov (clinical trial registration number: NCT03501602).
The parallel, randomised, clinical trial, with 1 : 1 allocation, was performed to evaluate the efficiency of different second-generation SADs and was carried out at the Health Sciences University Hospital Derince, Kocaeli, Turkey.
Eighty adult patients (ASA 1, 2 or 3) undergoing elective surgery under general anaesthesia were included in the study. Modified Mallampati scores, thyromental distance, the type of surgery, and mouth opening were recorded in the preoperative evaluation. Patients with expected difficult airway findings and BMI > 30 were excluded from the study. Randomization was allocated using www.random.org and stored in sealed opaque envelopes until consent was obtained. All patients received intramuscular atropine (0.5 mg) and midazolam (0.05 mg kg-1) as premedication 30 min before standardised induction of general anaesthesia.
Preoxygenation with 100% oxygen was applied for 3 min before general anaesthesia induction. Patients in the first and second groups were intubated with the LMA Protector (Teleflex Medical, Co. Westmeath, Ireland) (n = 40) and the i-gel (Intersurgical Ltd, Berkshire, UK) (n = 40), respectively. All patients were intubated by three senior anaesthesiologists with previous fibreoptic intubation experience. A 20 G peripheral IV cannula was inserted. Standard monitoring, including ECG, peripheral oxygen saturation, and non-invasive blood pressure, was performed. For induction of anaesthesia, IV propofol 2–3 mg kg-1, fentanyl 1–2 μg kg-1, and rocuronium 0.6 mg kg-1 were administered. The LMA Protector was inserted in the first group and the i-gel in the second group according to the manufacturer’s instructions. Size 4 and 5 SADs were used in men, and size 3 and 4 in women. The insertion time, number of attempts and complications were recorded.
The assessed complications were gastric content aspiration, hypoxaemia (SpO2 below 94%), upper airway trauma, laryngospasm, bronchospasm, laryngeal oedema, postoperative sore throat, and nerve injuries. Peak inspiratory pressure was kept below 20–25 cmH2O. Patients were ventilated with tidal volumes of 6–8 mL kg-1. When sufficient tidal volume could not be delivered, patients were ventilated by performing optimization manoeuvres [12]. These manoeuvres included jaw thrust, partial removal and reinsertion, and anterior traction on the tongue.
The oropharyngeal leak pressure was determined by closing the expiratory valve of the circle system using a gas flow of 3 L min-1. An audible noise was noticed when oropharyngeal leak pressure occurred in the circuit [13]. We attached the anaesthetic circuit to the swivel connector and performed continuous manual ventilation during the procedure. We loaded the Aintree catheter through the top port of the swivel connector into the i-gel or LMA Protector lumen. We then introduced the Storz Fiberscope (Karl Storz SE & Co. KG, Tuttlingen, Germany) with a loaded Aintree catheter. The fibreoptic scope’s outer and working channel inner diameters were 4.2 and 3.5 mm, respectively.
Brimacombe and Berry Bronchoscopy scores were determined by this fibreoptic scope as follows [14]:
vocal cords not visible;
vocal cords and anterior epiglottis visible;
vocal cords and posterior epiglottis visible;
only vocal cords visible.
Passing through the SAD, the carina was visualised, the bronchoscope was advanced to the front, and the Aintree catheter was left there. The insertion time for the SAD and Aintree catheter was recorded. First, the fibreoptic scope was withdrawn. Afterwards, we deflated and withdrew the SAD cuff. Then, we railroaded the tracheal tube over the Aintree catheter. An 8.0- to 8.5-mm-diameter tube was used for male patients; a 7.0- to 7.5-mm-diameter reinforced tracheal tube was used for female patients. During the procedure, systolic and diastolic blood pressure, heart rate, peripheral oxygen saturation, and end-tidal CO2 pressures were recorded.
Statistical analysis
Data obtained from the study were analysed using IBM SPSS Statistics 22. To compare the numerical data, the independent samples t-test and Mann-Whitney U test were used. The χ2 test was used to analyse the discrete variables. P-values of < 0.05 were assumed to be significant. We calculated the sample size of the study based on a preliminary study of 10 patients for each group. In the pilot study, the complication rates observed in the patients in the Protector and i-gel groups were 4/10 (40.0%) and 1/10 (10.0%), respectively. With a power of 80% (α = 0.05, β = 0.2) we determined that 32 patients in each group would suffice, and the study was completed with 40 patients in each group.
RESULTS
Three patients from each group were excluded from the study, and the data of the remaining 74 patients were analysed. In all patients excluded from the study, the reason was that adequate ventilation with the SAD could not be achieved. The ages of patients ranged between 19 and 82 years; the mean age was 42.41 ± 16.26 years. Forty-seven (63.5%) of the patients were women, and 27 (36.5%) were men. Data were collected in three different types of surgery including laparoscopic cholecystectomy in general surgery, discectomy in neurosurgery, and septoplasty in ear-nose-throat surgery (Figure 1). Demographic data did not differ significantly between groups (Table 1). The time and number of attempts at SAD insertion were similar between the study groups (Table 2). There was no statistically significant difference between groups in terms of Aintree catheter insertion time and tracheal intubation time, Brimacombe and Berry Bronchoscopy scores, and haemodynamic parameters. The airway complication rate was significantly higher in the LMA Protector group than in the i-gel group (21.6% vs. 2.7%, respectively, P = 0.013; Table 3). Hypoxaemia was recorded in one patient in the i-gel group. In the LMA Protector group, the complications were: bloody secretion on SAD due to upper airway trauma in three patients, bronchospasm in two patients, postoperative sore throat in two patients and a desaturation episode in one patient.
TABLE 1
Demographic data of the participants and the markers of difficult airway
TABLE 2
Supraglottic airway device insertion parameters
TABLE 3
Tracheal intubation parameters with Aintree catheter
In the evaluation of qualitative data, the need for optimization during SAD insertion, adequate tidal volume, and chest movement with SAD were statistically similar between groups. SAD insertion time, the number of attempts, Aintree catheter insertion time, and tracheal intubation time did not differ significantly between the groups (Table 2).
In the evaluation of quantitative data, no statistically significant difference was observed between the groups in terms of Mallampati score and thyromental and mouth opening distance (Table 1).
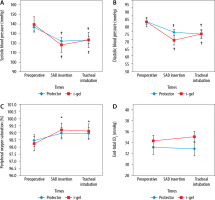
The changes in vital signs in both groups are summarised in Figure 2. Compared with preoperative values, there was a significant decrease in systolic and diastolic blood pressure and a significant increase in peripheral oxygen saturation. However, no statistically significant difference was found in the comparison between the groups. The mean (± SD) systolic blood pressure was 120.18 ± 18.08 and 123.41 ± 24.51 mmHg in the LMA Protector and i-gel groups, respectively (P > 0.05). The mean diastolic pressure was 72.20 ± 12.73 and 73.66 ± 14.45 mmHg in the LMA Protector and i-gel groups, respectively (P > 0.05). There was no significant difference between the groups in terms of end-tidal CO2 values (Figure 2) and oropharyngeal leak pressure.
FIGURE 2
Changes in vital signs in the two groups. A) Changes in systolic blood pressure in the two groups. B) Changes in diastolic blood pressure in the two groups. C) Changes in peripheral oxygen saturation in the two groups. D) Changes in end-tidal CO2 in the two groups *P < 0.05 indicates a significant increase in each group compared with the preoperative values, †P < 0.05 indicates a significant decrease in each group compared with the preoprative values. SAD – supraglottic airway device
DISCUSSION
In this study, two different SADs were compared for fibreoptic tracheal intubation. SAD insertion time, tracheal intubation time, need for optimization manoeuvre, and Brimacombe and Berry Bronchoscopy scores were similar. However, the complication rate was higher in patients in the LMA Protector group. Bronchospasm and bloody secretion on SAD were the most common complications.
Tracheal intubation through an SAD has been described by the Difficult Airway Society as a technique recommended for difficult airway management [4]. Studies on this issue are limited, and as stated in the guideline, there is no clinical comparative study using the Aintree catheter. In a study comparing 5 different SADs indirect fibreoptic intubation without using an Aintree catheter, the success rate of the i-gel was found to be 70% [2]. In all our patients who were excluded from the study, the reason was insufficient ventilation with the SAD. However, successful tracheal intubation was possible in all patients. The i-gel is often used successfully for fibreoptic intubation, including novice users, with its ease of insertion. In another study investigating direct fibreoptic tracheal intubation, the i-gel was compared with the Fast Trach, and all patients in the i-gel group were intubated at the first attempt [15]. Glottic visualization was found to be significantly better in favour of the i-gel. In our study, fibreoptic scope scores, SAD insertion time, and tracheal intubation time were similar between groups. However, our study with the Aintree catheter reveals that fibreoptic intubation can also be performed by novice users, because, in the event of desaturation or hypoxia during the procedure, it is possible to intervene through this catheter.
Kleine-Brueggeney et al. [16] compared the i-gel with the single-use ILMA for fibreoptic intubation, and similarly, the success rate of tracheal intubation was higher in favour of the i-gel. Also, airway leak pressure was significantly lower. Our study confirmed the high airway sealing pressures reported by other study groups for both SADs [17, 18]. In our study, no significant difference was found in terms of oropharyngeal leak pressures. This result suggests that the I-gel can be used successfully; however, we also concluded that the LMA Protector is not inferior in fibreoptic tracheal intubation.
It has been concluded in several studies that there may be difficulties during tracheal tube insertion, and these difficulties can be overcome with clockwise and anticlockwise manipulation [19]. However, this type of difficulty was not encountered in our study, and these manoeuvres were not needed. Moreover, no patient had oesophageal intubation. We believe that inserting a fibreoptic-guided Aintree catheter instead of blind endotracheal tube insertion reduces the frequency of complications. The most common complication of our study was bloody secretion on the SAD.
Complications occurred in eight patients in the LMA Protector group (21.6%), and six of them had bloody secretion on the SAD. In two patients, bronchospasm was observed. In the i-gel group, only one patient had bloody secretion on the SAD (2.7%). This ratio remained below the 5% value that appeared in the study of Kleine-Brueggeney et al. [16].
An investigation was performed in terms of intraoral laceration or additional complications in patients with observed blood, but no obvious pathology was determined. The LMA Protector is made of silicone material. The aim here is to make the SAD less stiff and soft and potentially reduce mucosal trauma when used in longer procedures [20]. Contrary to what has been advocated with this device, the incidence of trauma is high. We believe that this SAD, which has a more rigid structure compared with the i-gel, needs comparative studies in this regard. A recently published case report describing unilateral hypoglossal nerve palsy due to the LMA Protector supports the view that further studies should be conducted on the reliability of this device [11].
The importance of continuous ventilation during intubation through an SAD has been reported before [21]. In our study, continuous ventilation was provided through the swivel, which was recommended to be used with the Aintree catheter, and hypoxaemia was prevented during the procedure.
LIMITATIONS
The most important limitation of our study is that it was planned in patients with normal airway anatomy. This technique, which is recommended to be used in patients with difficult intubation, was 100% successful in our patient population; however, we are of the opinion that the expected difficult airway success rate may decrease. In this study, data were collected during different surgical procedures. It is a limitation that SADs have not been studied in the same types of surgery.