Introduction
High-energy cardiac implantable electronic devices (CIEDs) such as implantable cardioverter-defibrillators (ICD) or resynchronisation devices with cardioverter-defibrillators (CRT-D) implanted in both primary and secondary prevention of sudden cardiac death (SCD) prolong life [1, 2]. It is estimated that about 4.25 million deaths a year are due to SCD.
As was proven in the SCD-HeFT study (Sudden Cardiac Death in Heart Failure), in 21% of patients implanted with ICD for primary prevention of SCD, at least one adequate therapy of life-threatening arrhythmias was noted within 5 years after the implantation. In patients implanted for secondary prevention, the percentage of patients who were appropriately treated with ICD intervention was estimated in the AVID (Antiarrhythmics Versus Implantable Cardiac Defibrillators) trial at 69–85% within 3 years after the implantation.
The most appropriate intervention of ICD is limited to painless, and frequently unnoticeable by the patient, antitachycardia pacing (ATP) or a single shock. Nevertheless, there is a certain group of patients who receive multiple adequate interventions in a short period of time. Thanks to those interventions patients survive, but electrical instability and multiple discharges, defined as an “electrical storm” (ES), result in heart damage, exacerbation of heart failure and an increase in the hospitalization rate. Patients who have survived multiple ICD discharges have a significantly worse quality of life, suffer from depressive anxiety disorders and have 3-fold higher risk of death [1]. Thanks to ICD/CRT-D and remote monitoring systems, it is possible not only to detect and recognize ES, but also to shorten the time of reaction and apply the appropriate treatment. We may suppose that many cases of ES in patients with heart failure were lethal prior to the ICD/CRT-D era. Despite significant development of medicine, ES remains a very serious aggravating factor and 12-month mortality in patients with ICD who have survived ES is estimated at 33–54%. The term “electrical storm” refers only to the amount of ventricular arrhythmias and does not specify the condition of each patient. Some people are absolutely unaware of ES due to painless treatment of ATP and are diagnosed due to the remote monitoring transmission or during a routine follow-up in the outpatient clinic. Some come on foot, diverted for a check-up from emergency unit after a sudden fall and accompanying loss of consciousness. The most extreme cases are a nightmare for an on-call cardiologist – an unconscious patient in cardiogenic shock and clustering ventricular arrhythmias requiring urgent circulatory support or rescue ablation.
Bearing in mind how difficult it is to properly treat patients with ES and to decide which therapy and when it should be used, it seems to be impossible to plan and carry out a randomised trial assessing certain treatment patterns in such a diverse group. Therefore, the results from real life all-comers registries seem to be the best source to conclude which therapeutic procedures should be used and which group of patients should benefit most from a multidisciplinary approach. This article was written to analyse the most frequent causes of ES and prompt the most appropriate treatment for such patients.
Definition and epidemiology
The term “electrical storm” started to be used at the beginning of the 1990s to define the state of electrical instability of the heart manifesting as multiple and potentially lethal ventricular arrhythmias appearing in a short period of time [3].
In the current ESC guidelines ES is defined as > 2 episodes of ventricular tachycardia (VT) or ventricular fibrillation (VF) in 24 h [4]. In the current AHA guidelines regarding treatment of patients with ventricular arrhythmias and prophylaxis of SCD published in 2017, ES is defined as ≥ 3 episodes of sustained VT, VF or appropriate shocks from the ICD within 24 h [5].
Incidents should take place 5 min from each other to be considered separate episodes [6–8]. In patients with ICD/CRT-D ES is defined as ≥ 3 adequate detections of VT and/or VF in 24 h terminated with ATP or high voltage therapy (HVT), or untreated sustained VT recorded in the monitoring zone over 1 week after the implantation [8–11]. In most patients ES appears 2–3 years after the implantation [12, 13]. The incidence of ES in ICD recipients is estimated at 10–25% in 12 to 36 months after the procedure [6, 7, 13]. In primary prevention patients the incidence of ES reaches about 4% [12, 14, 15] and in secondary prevention 10–40% [6, 7]. Only a few analyses comparing the incidence of ES in patients with ICD and CRT-D have been performed [15–17]. The prevalence of ES in ICD vs. CRT-D groups was 7% vs. 0.6%, respectively, even though patients with CRT-D had significantly lower mean left ventricular ejection fraction (LVEF) when compared with ICD patients (21.7 ±11% vs. 34 ±15%) [15].
Nordbeck et al. reported that patients who positively responded to resynchronization therapy had a much lower incidence of ES (5.3% vs. 11.3%). This difference was explained by the beneficial influence of resynchronization therapy on the reverse modelling of the left ventricle [16]. Guerra et al. found that patients with CRT had a lower incidence of ES compared with propensity-matched ICD patients (5.6% vs. 12.3%) and CRT-D was associated with a 45% relative risk reduction in ES compared with ICD [17].
Mechanisms of ES
The most frequent arrhythmia causing ES is monomorphic VT (mVT) – 63–97% of cases [13, 17, 18]. This type of VT is usually caused by electrical activity in the transition zone located around scarring caused by previous myocardial infarction. Polymorphic VT (pVT) is less frequent (2–8%) and is more frequently caused by active myocardial ischaemia [14, 19].
Causes of ES
The ES is a very heterogenic sign of exacerbation of underlying disease such as heart failure, ischaemia, possibly reversible causes such as inflammation, electrolyte disturbances, hyperthyroidism or multiple factors combined, resulting in electrical instability and clustering ventricular arrhythmia. The ES is more frequent in the case of lower LVEF, in secondary prevention, exacerbated coronary artery disease (CAD), cardiac scarring and oedema in case of myocardial infarction or heart failure decompensation. Other possible factors mentioned in the literature predisposing to ES are: QRS duration (≥ 120 ms), infections with raised inflammatory markers, high levels of NT pro-BNP, electrolyte disturbances (mainly hypokalaemia), class I antiarrhythmic drugs according to Vaughan-Williams, stress or (CIED), and ES may be induced by stimulation of the right ventricle [20, 21]. Some reports can be found with results of small observational studies or a few case reports describing the occurrence of excessive alcohol consumption, in patients with cardiac implantable electronic devices of ES as a result of biventricular stimulation [22, 23]. In Table I possible reversible causes of ES are presented.
Table I
Most frequent reversible causes of ES
Treatment of electrical storm
The ES is a life-threatening condition and all patients should be hospitalized. Because of the very bad prognosis of patients with ES, it is advisable to divert such patients to well-specialised cardiology centres that offer a wide range of diagnostic and therapeutic approaches, especially invasive treatment and mechanical circulatory support. Even though patients with ES may be in different conditions – from unnoticeable events when VTs are terminated by ATP and arrhythmia is diagnosed by a remote monitoring system, to a severe condition with cardiogenic shock and multiple discharges of the ICD. It is proven that ES strongly increases the incidence of death, mainly secondary to worsening of heart failure [11, 16, 22].
Patients who have survived ES have a much worse quality of life and suffer from depressive and anxiety disorders [1].
In-hospital treatment
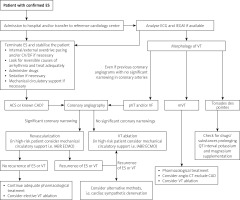
After admittance to hospital, the top priorities are to terminate clustering ventricular arrhythmias and to stabilise the patient while looking for reversible causes of ES. After performing the baseline clinical assessment, echocardiography and laboratory tests, different diagnostic pathways should be tailored according to the aetiology of heart disease and previous medical history of the patient. We strongly believe that the implementation of ES-algorithm treatment may be clinically effective. Therefore, a possible diagnostic and therapeutic algorithm (Figure 1) and treatment flow chart in case of persistent arrhythmia (Table II) have been proposed by the authors of this review.
Table II
Treatment of electrical storm in case of incessant arrhythmia
Programming of cardioverter-defibrillator to avoid unnecessary therapies
In some patients ES are treated only with ATP, which may remain unnoticed by the patients and be found due to the remote monitoring or during a routine follow-up in a cardiology clinic [24]. Unnecessary therapies are described as therapies delivered within a very short period of time from VT/VF onset, therefore, preventing non-sustained VT/VF from self-terminating. Aggressive ICD programming has already been associated with increased all-cause mortality and potentially could contribute to an increased incidence of ES [25]. It is documented that each HVT increases the risk of death by 20% when compared with the group of patients with VT terminated by ATP [11]. The efficacy of ATP in VT up to 250/min reaches 81% [26–29].
Pharmacotherapy and stabilization of the patient’s condition
One of the most effective and helpful ways to terminate ES is the tension reduction of the sympathetic system by β-blockers and tranquilizers (mainly benzodiazepines). β-Blockers are drugs of first choice, but their effect is frequently insufficient and needs to be associated with other antiarrhythmic drugs [30]. Amiodarone is one of the most efficient drugs in the treatment of ventricular arrhythmias, especially when combined with a β-blocker [31]. According to current guidelines, intravenous amiodarone therapy is recommended in case of pVT [30]. Sotalol was proved to decrease the amount of ICD discharges and death by 44% [32]. On the other hand, the SWORD (Survival With Oral d-Sotalol) trial showed that sotalol increased the risk of death in patients with heart failure and led to HF decompensation, and it should not be used in patients with LVEF lower than 40%. In case of contraindications or unsuccessful treatment with drugs mentioned above, lidocaine infusion may be used in the acute phase of ES (especially in the case of concurrent ACS) [4, 30, 33]. Mexiletine is an antiarrhythmic drug which can be used in the acute phase of ES, lowering the incidence of VT/VF clusters in case of insufficient treatment with amiodarone. Due to the high rate of side effects, mexiletine is used in short-term therapy only [34].
Invasive approach
Most patients with ES have CIEDs implanted mainly because of heart failure (HF). Seventy percent of cases of HF are caused by the left ventricle function deterioration induced by ischaemia. Bearing that in mind together with the fact that ES is a life-threatening condition and the prognosis of survivors is very poor, invasive procedures – diagnostic (such as coronary angiography or electrophysiological study) and therapeutic (percutaneous coronary intervention, surgical revascularization, ablation or other methods) – should always be considered. In many cases and due to electrical and haemodynamic instability, and/or patient’s characteristics, the above-mentioned procedures are of high risk and require circulatory support. In recently published papers evaluating clinical outcomes of patients receiving haemodynamic support (HS) during ventricular tachycardia ablation it was shown that patients requiring HS were sicker, had multiple comorbidities and had a significantly higher 1-year mortality than patients in the no-HS group. In patients with LVEF ≤ 20% and NYHA class III to IV, there was no significant difference in clinical outcomes when compared with the no-HS group. Investigators underline that further studies are necessary to evaluate patients undergoing VT ablation with HS [35]. The authors of this article postulate that all of the methods mentioned above should be treated as complementary. Clinicians, electrophysiologists, intensive coronary unit (ICU) cardiologists, invasive cardiologists and cardiac surgeons should participate together in the diagnostic and therapeutic process of patients with ES. This kind of multilevel and multidisciplinary approach may be beneficial and translate to the improvement of clinical efficacy. An Electrical Storm Team consisting of the above-mentioned specialists should be a structure present in the centres of the highest reference.
Revascularization
The recently published results from the Sudden Cardiac Death in Patients With Ischemic Heart Failure Undergoing Coronary Artery Bypass Grafting (STICH) trial indicate that the monthly risk of SCD shortly after CABG among patients with a low LVEF is the highest between the first and the third month. In such patients, especially those with an increased perioperative end-systolic volume index and/or B-type natriuretic peptide, the risk stratification for SCD should occur early in the postoperative period [36].
According to the authors of this article, all patients with ischaemic etiology of HF or previously observed artherosclerotic changes in coronary arteries (even non-significant at the time of the angiogram), should be referred for a coronary angiogram (CA) and undergo complete revascularization (percutaneous of surgical) in case of finding a significant narrowing. The existence of possible reversible causes of ES in such patients does not exclude coexisting ischaemia resulting from the progression of arteriosclerosis of the coronary arteries. Such an approach in ES, even though not supported by the evidence coming from the documents available, seems to be clinically justified and intuitive. Both guidelines on myocardial revascularization from 2014 and those published in 2018 state that urgent CA and revascularization should be a part of management of patients with ES (class II a, C), but neither of them describes precisely which patients should undergo such a procedure and no time intervals were established [37, 38]. As long as there are no trials dedicated to ES, decisions regarding timing, ways of revascularization, spectrum, usefulness of additional examinations such as fractional flow reserve, intravascular ultrasound, optical coherence tomography, viability assessment and so on, should be based on current guidelines regarding revascularization and other studies assessing the prognosis of patients with heart failure with or without complete revascularization [38, 39].
According to current AHA guidelines, revascularization is a successful technique in reversing myocardial ischaemia which is a cause of sustained polymorphic VT or VF [5].
Patients who present with VF or polymorphic VT in the postoperative period more often have associated ischaemia, while patients presenting with monomorphic VT usually have an old infarct and ventricular scar [2]. Polymorphic VT/VF occurring after CABG warrants a therapeutic approach targeting the treatment of myocardial ischaemia, including a possible need for assessment of graft patency, as well as the identification and treatment of mechanical complications and acute electrolyte or acid base disturbances. The risk factors for the occurrence of monomorphic VT early after CABG include prior MI, ventricular scar, LV dysfunction, and placement of a bypass graft across a non-collateralized occluded coronary vessel to a chronic infarct zone [5]. Additionally, it is likely that successful revascularization may improve the effectiveness and safety of the planned ablation.
Most patients with ES have an ischaemic background of cardiomyopathy, and therefore it is likely that the ventricular arrhythmia is triggered by myocardial ischaemia [40]. When ES is associated with ACS, guidelines for this condition should be followed. One of the impediments to establish a proper diagnosis may be the fact that patients with ES may develop changes in ECG patterns and myocardial ischaemic markers may be raised as a consequence of arrhythmia and multiple discharges of the ICD and not by ischaemia per se. Until now, there is no evidence from randomized trials assessing the benefits of prophylactic coronary revascularization in patients hospitalized because of lethal ventricular arrhythmias or in survivors of sudden cardiac arrest (SCA) secondary to VT/VF without concomitant ACS. Published clinical evidence suggests that myocardial ischaemia is a vital factor in case of SCA or adequate intervention of the ICD and indicates that prophylactic revascularization of coronary arteries may lower the ventricular arrhythmia burden in those patients [41]. Intentional and complete revascularization of coronary arteries is indicated in patients in whom myocardial ischaemia may be present and is likely to cause recurrent VT/VF and in people in whom an underlying ischaemic aetiology of ventricular arrhythmia cannot be excluded [30]. It was proved that revascularization of significantly narrowed coronary arteries decreases the incidence of arrhythmia recurrence [42]. One third of patients with significant coronary disease have chronic total coronary occlusion (CTO), which is associated with long-term mortality in patients with previous myocardial infarction together with a high risk for ventricular arrhythmias [43]. Another small study showed that a CTO in an infarct-related artery (IRA-CTO) is an independent predictor of VT recurrence after ablation and identifies a subgroup of patients with a high recurrence rate despite a successful procedure [44].
Ablation
Patients with ischaemic cardiomyopathy and an ICD who had ventricular tachycardia despite antiarrhythmic drug therapy and undergoing catheter ablation have a significantly lower cumulative rate of death, ES or appropriate ICD shock than patients with increased doses of antiarrhythmic drugs [45].
The VT ablation is indicated in case of insufficiency of pharmacotherapy, lack of reversible causes of ES and, together with revascularization, in patients with ES. It is proved that VT ablation in patients with ES significantly decreases the recurrence of ventricular arrhythmia and, in combination with optimal pharmacotherapy, may prolong life in those patients [46].
Radiofrequency ablation (RF) has limited effectiveness in treatment of VT. Thanks to the introduction of new techniques combined with electroanatomical mapping such as CARTO or EnSite, the efficacy of ablation has increased [47]. The ES more frequently affects patients with severely decreased LVEF (mean of about 27–30%) and in such cases may be of high risk. The results of extracorporeal membrane oxygenation (ECMO) support during catheter ablation of unstable VT are encouraging. After a median follow-up of 21 months (13–28 months), VT recurrence was 33% and overall survival was 56 out of 64 (88%) patients. The ablation of unstable VTs supported by ECMO allowed rhythm stabilization with low procedure mortality together with bridging decompensated patients to a permanent left ventricular assist device (LVAD) or heart transplantation [48]. However, this does not seem to translate into significant long-term benefits in terms of arrhythmia-free survival or mortality [49].
Other interventional methods of treatment
In patients with ES in whom pharmacological treatment and catheter ablation are ineffective or not possible, cardiac sympathetic denervation (CSD) may be an option [24, 25].
The CSD may lead to effective control of the arrhythmic burden in up to 56% of patients [50]. In a recent multicentre registry that included 121 patients with structural heart disease who underwent left or bilateral CSD for refractory VT or ES, bilateral CSD was associated with a two-fold risk reduction of the combined event of sustained VT/ICD shock recurrence, death, and/or heart transplant as compared with patients who underwent a left side-only procedure [51]. In case of incessant ventricular tachycardia storm, especially resulting in cardiogenic shock, an intra-aortic balloon pump (IABP), mechanical circulatory support with percutaneous ventricular assist devices (pVAD) such as extracorporeal membrane oxygenation (ECMO), TandemHeart, and Impella, or an LVAD may be considered in order to increase systemic blood flow, protect against organ hypoperfusion and protect the myocardium through a decrease in oxygen consumption [52, 53]. Even though there are insufficient data regarding those methods, it seems to be a good way to stabilise patients and provide a safe bridge to invasive target treatment or heart transplant for the most sick patients with ES.
Future perspectives
There is an ongoing international study called ELECTRA, with two main aims of the study defined by its authors: to create an international registry on ES containing information about clinical features, pharmacological management and interventional treatment strategies, and to use the data derived from the registry to describe mortality and rehospitalization rates over a long follow-up in patients with ES [54].
Table III sums up the most important studies describing the effects of invasive treatments in patients with ES, with some alternative approaches in high-risk patients with ventricular arrhythmias uncontrollable with antiarrhythmic drugs and standard methods [55–65].
Table III
Summary of most important studies describing invasive treatment of electrical storm and assessing effectiveness of each procedure
| Author | Year of publication | Number of patients, n | Time of observation | Revascularization in ES | Ablation in ES | Sympathetic denervation | Results and main findings |
|---|---|---|---|---|---|---|---|
| Carbucicchio [47] | 2008 | 95 | 22 months (median) | No | Endo- and epicardial (in 10 patients) | No | |
| Koźluk [55] | 2011 | 24 | 27.8 months (mean) | No | Yes | No | |
| Ajijola [50] | 2012 | 6 | 9–28 days | No | Yes (bilateral) | ||
| Viswanathan [42] | 2013 | Literature review | Yes, CABG and PCI | No | No | ||
| Bella [56] | 2013 | 528 | 26 months (median) | No | Yes | No | |
| Hofferberth [57] | 2014 | 24 | 28 months (median) | No | No | Left thoracoscopic sympathectomy | |
| Di Marco [44] | 2015 | 191 | 19 months (median follow-up) | No | Yes | No | |
| Kumar [58] | 2015 | 67 | 6 months | No | Transcoronary ethanol ablation, surgical epicardial window or surgical cryoablation | No | |
| Sapp [45] | 2016 | 259 | 27.9 months (median) | No | Yes | No | |
| Saenz [59] | 2016 | 75 | 7 months (median) | No | No | Yes, bilateral | Amount of ICD shocks: decreased from 4 (2–30) to 0 (0–2) |
| Baratto [48] | 2016 | 64 | 21 months (median) | No | CA with ECMO support | No | |
| Santangeli [60] | 2016 | Systematic review: 2268 from 8 trials assessed antiarrhythmic drugs (AAD), 427 from 6 trials assessed catheter ablation (CA) | 15 and 14 months (median), respectively | No | Yes | No | |
| Muser [49] | 2017 | 267 | 45 months (median) | No | Yes | No | |
| Vaseghi [51] | 2017 | 121 | 1.1 years (median) | No | No | Yes | |
| Meng [61] | 2017 | Systematic review: 38 patients from 23 studies | No | No | SGB | ||
| Le Pennec-Prigent [62] | 2017 | 26 | 34.7 days (median) | No | No | No | |
| Vergara [63] | 2018 | 1940 | 12 months | No | CA | No | |
| Enriquez [64] | 2018 | 21 | 10 days (median) | No | CA with ECMO support (patients in CS) | No | |
| Sierpiński [65] | 2018 | 101 | 22.8 months (median) | No | Yes | No |
[i] AAD – antiarrhythmic drugs, ACS – acute coronary syndrome, AHD – acute hemodynamic decompensation, CA – catheter ablation, CS – cardiogenic shock, CSD – cardiac sympathetic denervation, ECMO – extracorporeal membrane oxygenation, ES – electrical storm, ICD – implantable cardioverter-defibrillator, ICM – ischemic cardiomyopathy, IR – confidence interval, IRA-CTO – infarct-related artery chronic total occlusion, NIDCM – non-ischemic dilatative cardiomyopathy, OHT – orthotopic heart transplant, OR – odds ratio, SGB – stellate ganglion block, VA – ventricular arrhythmia, VT – ventricular tachycardia.
Follow-up for es survivors
The ES survivors need careful and systematic control as a group of very poor prognosis of survival and are more prone to subsequent dangerous ventricular arrhythmias.
Remote monitoring is a very useful tool in all CIED patients, but of vital significance in patients with a history of ES. It not only provides information about arrhythmias, signs of heart failure worsening, shortens time of reaction and assesses the percentage of biventricular stimulation, but also lowers patients’ anxiety and improves the sense of security, therefore improving the quality of life.
Systematic follow-up visits in the clinic allow one to supervise patients’ condition, assess the efficacy of pharmacological treatment and its modification, allow one to assess progress of heart failure and CAD exacerbation and early recognition of unnoticed ventricular arrhythmia (sustained and not sustained) as an early sign of electrical instability.
Practical approach from clinical experience
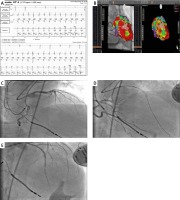
To underline the importance of a multidirectional approach to treatment of ES, we would like to present a clinical case of a patient treated in our hospital due to ES. A 72-year old man with diabetes mellitus, arterial hypertension, heart failure after anterior wall Q-wave myocardial infarction treated conservatively (in 1987), with a history of PCI with a drug-eluting stent of the RCA in 2014 and after ICD implanted in primary prevention of sudden cardiac death, was referred from a remote monitoring unit directly to admission to our centre due to electrical storm (in summary: 27 episodes of VT adequately treated with ATP and ICD discharges during the last 3 days, Figure 2 A).
Remote monitoring of patients with HF and CIED may improve long-term prognosis [66], with a shorter time from diagnosis to medical action, as in this particular case.
On admission the patient presented with no signs of possible acute coronary syndrome (no stenocardia or ECG changes, necrosis markers negative) or significant heart failure deterioration. Left ventricle ejection fraction was 25% with akinesia of the anterior wall. Control coronary angiography revealed good patency in the previously stented RCA and totally occluded LAD (already observed on coronary angiogram in 2014). According to the heart-electrical team decision, VT ablation was performed (Figure 2 B).
Unfortunately, after 2 days recurrence of the sustained, haemodynamically unstable VT was observed. Considering that the presence of the CTO in HF patients significantly affects the long-term prognosis [39] and on the basis of maps of potentials recorded during VT ablation (border zone adjacent to aneurysm showing low potentials and partial viability) we decided to open the chronically occluded LAD (Figures 2 C–E). During 12-month follow-up VT recurrence was not recorded.
Conclusions
The number of patients with cardiac implantable electronic devices is rising. One of the most severe and challenging conditions in these patients is the electrical storm; therefore, it seems to be crucial to be aware of possible reversible causes, alternatives for treatment and useful algorithms of investigation and treatment: conservative and, equally importantly, interventional. Mortality among the survivors of electrical storm is very high; hence treatment should be of broad spectrum, tailored for each patient and involving both the acute phase of electrical instability and outpatient follow-up.