Introduction
The detection and use of BRAF inhibitors, and later MEK inhibitors, was one of the breakthroughs in the treatment of advanced melanoma. At first, these drugs were only used to treat patients with inoperable locally advanced or metastatic melanoma. Subsequently, based on the Combi AD clinical trial, dabrafenib with trametinib was also registered for adjuvant treatment after resection of regionally advanced melanoma. The results of the aforementioned study showed that the use of dabrafenib with trametinib (the only type of BRAF and MEK used in adjuvant treatment) in stage III patients undergoing radical surgery reduces the risk of recurrence by 16% at 4-year follow-up. Complementary treatment with dabrafenib with trametinib is now one of the standards for management of patients with the current mutation in the BRAF V 600 E/K gene, after prior radical surgical management [1].
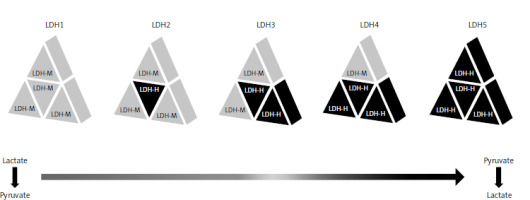
One of the best known and commonly used biomarkers for melanoma patients is serum lactate dehydrogenase (LDH) level. Lactate dehydrogenase is an enzyme commonly found in cells, playing a major role in aerobic glycolysis. It is a tetrameric molecule composed of LDH-H and LDH-M subunits, which are encoded by the LDH-A and LDH-B genes. There are 5 isoforms of the enzyme in question (Fig. 1). The isoforms consisting mainly of LDH-M, i.e. mainly LDH 5 and to a lesser extent LDH4, preferentially catalyse the pyruvate to lactate transition step, while the isoforms consisting mainly of the LDH-H subunit, i.e. lactate dehydrogenase 1 and 2, reverse the reaction. Lactate dehydrogenase isoforms are not commonly analysed in blood serum, but it is known from scientific research that the LDH-5 isoform is predominant in cancer, and its presence is related to a worse prognosis. What is very important is that LDH is a cytosolic enzyme that enters the blood only when the cell membrane is damaged. Lactate dehydrogenase concentrations in serum do not always reliably reflect concentrations in tumour cells. A study in melanoma patients showed that high glucose uptake by 18-F-fluorodeoxyglucose-positron emission tomography was associated, but not fully overlapping, with elevated serum LDH levels [2–6]. The information that the cell membrane must be damaged to release LDH into the serum indicates that the above process is exacerbated when there is so-called increased cellular turnover, i.e. when there are more cells dividing and consequently undergoing necrosis.
Lactate dehydrogenase concentration affects the qualification of metastatic disease progression. It also has prognostic significance. Its increased activity correlates with a lower response rate to the therapies used (both targeted therapy and immunotherapy) and is related to a shorter survival time [7, 8]. However, most importantly, an increase in LDH may be the first sign of disease recurrence after radical melanoma resection. In such a situation, LDH acts as a diagnostic biomarker [8]. According to our observations, LDH loses its designation as a diagnostic biomarker indicating disease recurrence during follow-up therapy with dabrafenib with trametinib.
Material and methods
The main objective of the study was to analyse the concentration of LDH at various time points in patients undergoing adjuvant target therapy.
Among stage III melanoma, BRAF-mutated patients undergoing radical surgery followed by adjuvant therapy at the Department of Clinical and Experimental Oncology in Poznań received combination treatment with dabrafenib and trametinib. Treatment was carried out in accordance with current standards of therapeutic management of melanoma patients and bore no features of a medical experiment. Patients gave informed verbal and written consent to the proposed treatment prior to the start of treatment. A computed tomography scan was performed before the treatment to exclude melanoma spread. Thereafter, this scan was performed every 12 weeks or when a recurrence was suspected. Patients undergoing targeted therapy reported to the centre every 4 weeks, where laboratory tests such as complete blood count with smear, aminotransferases, creatinine, amylase, electrolyte evaluation, and LDH were performed before the drugs were dispensed. During the first treatment period, the tests were performed before each treatment cycle, and from the fifth cycle onward every 2 months. Treatment was continued for 12 months or until disease progression or unacceptable toxicity was noted.
Results
Out of the 23 patients discussed, 21 completed treatment as planned. One patient had treatment discontinued due to unacceptable toxicity. One patient terminated treatment due to melanoma recurrence at 8 months of therapy. Another 6 patients had recurrence of melanoma ranging from 21 to 35 months after starting treatment with dabrafenib and trametinib and were treated with immunotherapy. After a follow-up period of, respectively, 1.5–5.5 years, 21 patients are alive.
The upper limit of LDH norm in a local laboratory is 225 UI. Lactate dehydrogenase increase was observed asymptomatically in all 23 patients during treatment. An increase in LDH and its persistence above the upper limit of normal was observed in 18 of them, while it was within normal limits in 4. After discontinuation of dabrafenib with trametinib, a decrease in LDH levels was observed in all patients except one, in whom treatment was discontinued due to disease progression. Decreases in LDH values were also observed in patients who had their treatment interrupted due to adverse events. In these patients, when they returned to treatment, there was again an increase in LDH (Fig. 2). The range of the increase in LDH levels was 32–193 UI, which was 18–153% of the baseline value. The median increase in LDH levels in all patients was 96 UI, representing 53% of the baseline value. The increase in LDH was not accompanied by leukocytosis, and no increase in neutrophil to leukocyte ratio (NLR) inflammatory index was observed between the baseline values and the values at maximum increase in LDH. On the contrary, the median NLR of all the patients in question decreased by 0.86 between the given time points. In addition, a decreasing trend was observed for the number of leukocytes and neutrophils. Also, a decreasing trend for the number of leukocytes and neutrophils was observed in 19 patients. No significant differences were noted for haemoglobin concentration and platelet count. C-reactive protein was not determined as a standard.
Discussion
The mechanism of the effect of BRAF and MEK inhibitors on the increase of LDH in the serum of patients has not been described so far. In the following, several possible hypotheses that may explain the above phenomenon are posed. However, the above results require their confirmation in a larger group of patients.
The first important element necessary to explain the analysed phenomenon is the difference in the effect of inhibitors on cells with the present BRAV V600 mutation and cells without the mentioned mutation.
Mutations involving points in RAS-RAF-MEK-ERK signal transduction pathway have become an important therapeutic target for many targeted therapy strategies. Activating mutations in BRAF genes are described in 40–50% of melanoma cases, respectively. As is well known, BRAF inhibitors are responsible for inhibiting the pathological stimulation of the above pathway in patients with the present mutation in the BRAF V600 gene [9–12]. At the same time, information about the stimulatory effect of BRAF inhibitors on the MAPK pathway in wild-type free cells is important; BRAFi induces paradoxical activation of the MAPK pathway through a conformational change of the BRAF protein, which promotes dimerisation and interaction with RAS. This results in allosteric transactivation and phosphorylation of the RAF dimer, which leads to further signalling, thereby hyperactivating the MAPK pathway [13, 14].
The above reports indicate that it is possible that activation of the MAPK pathway may be paradoxically stimulated in cells that do not have BRAFV600E/K mutation, and thus increase so-called cellular turnover, which may lead to an increase in LDH levels.
However, it should be noted that during targeted therapy with BRAF and MEK inhibitors of patients with active, advanced melanoma, when treatment-induced elevated LDH levels result from increased tumour glycolytic activity and necrosis, a decrease in LDH levels is observed in the vast majority. At the same time, in the above situation, in accordance with the hypothesis presented earlier, there should also be a paradoxical activation of MAPK pathway in cells without mutations in the BRAF gene and a partial increase in LDH. It may be that the LDH concentration is then the resultant of both situations presented.
Another hypothesis, which in our opinion can be explained by the described phenomenon, is the immunomodulatory effect of drugs in question. There are several reports indicating immunostimulatory effects of both BRAF inhibitors and MEK inhibitors. The known mechanisms of action involve reduction of immunosuppressive cell populations and reduction of immunosuppressive cytokine production, as well as stimulation of cytokine and immunostimulatory molecule production. Importantly, the above actions are described for the combination of BRAFi and MEKi in melanoma patients with a mutation in the BRAF gene present, and for MEKi alone in wtBRAF melanoma patients. BRAF and MEKi increase the expression of tumour antigens and the expression of HLA I and/or HLA II, and increase T-cell reactivity and cytotoxicity in BRAF-mutated cells, and MEKi alone analogously in BRAF wild-type cells [13, 15]. Activation of the immune system is also related to increased cellular turnover and consequently increased release of LDH from the cytoplasm.
Conclusions
An increase in LDH levels during complementary therapy with BRAF and MEK inhibitors is a common phenomenon. In relation to the above, the increase in LDH cannot be used to detect a possible recurrence of melanoma during the treatment in question and, in our opinion, is not an indication for additional imaging studies. The presented observation needs to be confirmed in a larger patient population, and if these results are confirmed, routine LDH determination during adjuvant therapy with BRAF and MEK inhibitors seems pointless. In addition, our observation avoids unnecessary exposure of patients to X-rays, emotional stress, and additional economic burden on the health care system. To evaluate the effect of individual inhibitors on LDH increase, it would also be most appropriate to expose patients only to BRAFi or only to MEKi, but for ethical reasons this seems impossible at present. An analysis of LDH isoforms that undergo an increase during the therapy in question would also be important, requiring further study.