Introduction
Neoplasms of the lungs are the leading cause of cancer incidence and mortality worldwide [1]. Lung cancer is divided based on the cell of origin into small-cell lung (SCLC) and non-small-cell lung cancers (NSCLC), the latter of which is further divided. According to the 2015 WHO classification, the most common types of lung cancer include adenocarcinoma (cancer of glandular cells), squamous cell carcinoma (SCC), and neuroendocrine cancers such as small cell carcinoma (SCLC), large cell neuroendocrine carcinoma (LCNEC), and carcinoid [2]. Carcinoid tumorstumours are cancers of well-differentiated neuroendocrine cells (Kulchitsky cells), while SCLC also arises from poorly differentiated neuroendocrine cells, resulting in rapid metastasis, and poorly responseive to therapy, and poor prognosis. Squamous cell and small cell cancers are more likely to be centrally located and associated with a history of smoking, especially among men. Adenocarcinoma is more likely to arise in women and those without a smoking history, they arise peripherally, and test positive for targetable driver mutations such as epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), BRAF, and ROS1. Receptor tyrosine kinase small molecule inhibitors against these mutations, as well as immunotherapies such as programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors, have in recent years replaced or supplemented chemotherapy in eligible patients [3]. Over 80% of lung cancer cases in the Western world are attributable to smoking, and progress in smoking cessation has resulted in decreases in incidence and mortality. Continued smoking, as well as further risk factors such as occupational exposure to asbestos and combustion fumes, as well as environmental exposure to arsenic and air pollution, remain major contributors in the developing world. A stronger understanding of lung cancer epidemiology and risk factors can inform preventive measures and curb the growing disease burden worldwide.
Epidemiology
Incidence
According to the latest GLOBOCAN estimates, 2,094,000 new cases of lung cancer were diagnosed globally in 2018, making lung cancer the leading cancer incidence worldwide. With an estimated 1,369,000 cases, lung cancer is the second most common cancer in men, after prostate cancer, and the second most common cancer in women, after breast cancer, with 725,000 cases. The age-standardized cumulative lifetime risk of diagnosis of lung cancer is 3.8% among men and 1.77% among women [4, 5].
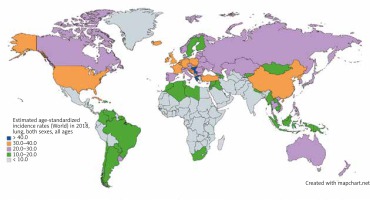
Lung cancer has the highest incidence in developing nations where cigarette smoking is most prevalent, with over a 20-fold variation in incidence between regions. While prostate cancer is the most common cancer among men in 104 nations, lung cancer is the most common in 37 nations, including Russia, China, and much of Eastern Europe, the Middle East, and Southeast Asia (Fig. 1) [5]. Lung cancer is the most common cancer among women in 1 nation: North Korea. Micronesia/Polynesia is the region with the highest incidence of lung cancer worldwide at 52.2/100,000 cases among men, while Hungary is the nation with the highest incidence at 77.4/100,000 among men. Among women, North America and Northern and Western Europe have the highest incidence worldwide. Western, central, and eastern Africa have the lowest incidence among men and women [4].
Fig. 1
Map showing estimated age-standardized incidence rates for lung cancer worldwide in 2018, in both sexes including all ages. Created with mapchart.net. Data obtained from Globocan 2018 [5]
According to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program, there were an estimated 229,000 new cases of lung cancer in the US in 2020, accounting for 12.7% of all cancer diagnoses. The current incidence of 45.6/100,000 is down from a peak of 69.5/100,000 in 1992, largely due to smoking cessation. While many Western nations report a similar trend, developing nations such as China and the nations of the former Soviet Union have not seen similar success with smoking cessation and lung cancer incidence [6]. In China, 65% of men currently initiate smoking by their mid-20s, portending a continued increase in lung cancer incidence for decades to come [7]. With increased industrialization and access to tobacco worldwide, the worldwide incidence of lung cancer is on the rise [8].
Mortality
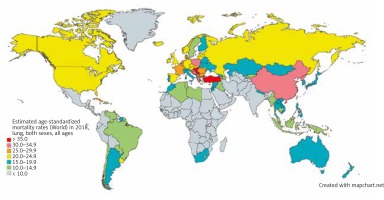
Lung cancer is the leading cause of cancer mortality worldwide, among both sexes and men and women separately. In 2018, lung cancer accounted for an estimated 1,761,000 deaths, 18.4% of all cancer deaths worldwide. With an estimated 1,185,000 deaths in 2018, lung cancer was the leading cause of death among men in 93 nations, including the US, Russia, and China (Fig. 2) [5]. Women were less than half as likely to die of lung cancer as men, with an estimated 576,000 deaths among women in 2018. Lung cancer was the leading cause of death in women in 28 nations, including the US and China [4].
Fig. 2
Map showing estimated age-standardized mortality rates for lung cancer worldwide in 2018, in both sexes including all ages. Created with mapchart.net. Data obtained from Globocan 2018 [5]
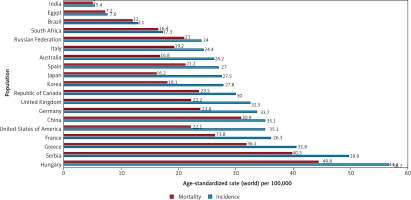
Unlike in men, lung cancer incidence and mortality among women in the US and Europe continue to rise. In 2017, lung cancer surpassed breast cancer as the leading cause of cancer death among women in Europe, with 14.6 deaths per 100,000 women [9]. A comparison of the incidence and mortality rates of different countries is provided in (Fig. 3) [5].
Fig. 3
Bar chart showing estimated age-standardized incidence and mortality rates (World) in 2018, lung, both sexes, all ages. Data obtained from Globocan 2018 [5]
Survival
According to SEER, the latest 5-year survival rate for lung cancer in the US (from 2010–2016) was 20.5%. The earliest reported 5-year survival rate, in 1975, was 11.5% [6]. Increases in survival are likely due to earlier detection (e.g. recommended computed tomography (CT) screening among those with significant smoking history), as well as improvements in treatment modalities with the introduction of targeted (EGFR, ALK, ROS, and BRAF inhibitors) and immune therapies (PD-1, PDL-1, and CTLA-4 inhibitors). Higherincome nations have better access to the latest diagnosis and treatment tools and better lung cancer survival [8].
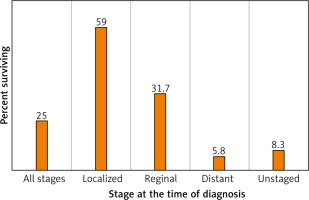
In the US, the 5-year survival for those with localized disease at diagnosis (stage I-II) is 59.0%, decreasing to 31.7% among those with regional (stage III) disease and 5.8% among those with metastatic (stage IV) disease (Fig. 4). 57% of lung cancer cases in the US are diagnosed after metastasis [6].
Fig. 4
Lung and bronchus cancer recent trends in SEER relative survival rates, 2000–2016 by sex, all races (includes Hispanic), all ages, all stages, 5 years [6]
Non-modifiable risk factors
Age
The average age for lung cancer diagnosis in the US was 70 years old among men and women. An estimated 53% of cases occur in those 55–74 years old, while the remaining 37% occur in those > 75 years old [10]. Lung cancer is the leading cause of death in men over 40 years old and in women over 59 years old in the US [11].
Tumourigenesis in the lungs seems to occur decades after initial exposure to mutagens, explaining the delayed time course of the disease. Biological aging also probably contributes because, with age, telomeres shorten, levels of the metabolite NAD+ decline, and cells lose the ability to withstand and repair DNA damage as well as surveil for aberrant cells [11–13].
Lung cancer can also be seen in younger adults, with patients under 55 years old accounting for 10% of cases in the US. Studies of NSCLC in those aged 20–46 years find that younger patients tend to be female, non-smokers, and present with more advanced adenocarcinoma, suggesting a disease course more associated with heritable mutations and less associated with environmental mutagens. Young patients across stages and lung cancer subtypes are more likely to receive aggressive treatment and report better survival [14].
Gender
Globally men are over doublemore than twice as likely to be diagnosed with, and die of, lung cancer. The largest contributor to the gender disparity is that men are more likely to smoke tobacco. In the developed world, lung cancer rates are falling for men while rising for women, becauseas women were later to historically adopt, and then cease, tobacco smoking [15]. Transgender men and women in the US currently have a higher prevalence of cigarette smoking compared to the national average (35.5% vs. 13.7%) [16, 17].
Whether women are innately more susceptible to lung cancer remains contentious. There is a higher rate of lung cancer in non-smoking women compared to non-smoking men, specifically EGFR positive adenocarcinomas [18, 19]. Women tend to be more likely to have other predisposing mutations such as those involving the CY1A1 and TP53 genes, and tend to carry a higher familial risk, irrespective of smoking status. ALK gene rearrangements and BRAF gene mutations are more common in women than in men with NSCLC [19, 20].
Hormonal influence has likewise been suggested to contribute, with a oestrogen receptors (OR) found to be overexpressed in adenocarcinomas, and anti-oestrogen compounds showing anti-tumour effects in vitro [21]. Variables affecting oestrogen exposure, such as length of the menstrual cycle, age at menarche and menopause, and parity, have shown varying strengths of association with lung cancer risk [19, 22]. Hormonal replacement was found to have no significant risk of lung cancer in a meta- analysis of 14 cohort studies [23].
Race/ethnicity
In the US, African American men have the highest incidence of lung cancer (87.9/100,000), while Hispanics and Asians/Pacific Islanders have the lowest incidence rates (45.2 and 40.6, respectively). Among women, Caucasian Americans have the highest incidence (57.6/100,000), while African American women are slightly lower at 50.1, and Hispanics and Asians/Pacific Islanders are less than half that (25.2 and 27.9, respectively) [11].
While cigarette smoking accounts for the greatest variation across ethnicities and nations, other genetic and environmental factors are likely to contribute [15]. Chinese women are equally likely as Western European women to be diagnosed with lung cancer, despite having a significantly lower rate of reported cigarette consumption. Exposure to smoke from charcoal burning and air pollution are thought to contribute to the increased risk among Chinese women [4]. When compared to western (Caucasian) patients with NSCLC, East Asian patients have a much higher prevalence of EGFR mutations, predominantly among patients with adenocarcinoma and never-smokers [24]. Han Chinese and Japanese populations have been found to have mutations in the 3q28 locus associated with increased lung cancer risk [25]. Similar genetic alterations that modulate lung cancer risk have been described to vary in prevalence among racial and ethnic groups [26].
Disparities in lung cancer survival between races have also been documented, with minorities and low-income patients in the US less likely to receive aggressive therapy for lung cancer [27], and less likely to be enrolled in clinical trials [28].
Family history
Discrepant lung cancer presentation among tobacco smokers and non-smokers suggests a heritable component to lung cancer risk. A positive family history increases the risk of lung cancer by 1.7 times, according to metaanalyses from Central and Eastern Europe [29]. If the history is among first-degree relatives, the risk is increased to 2–4 times, even when controlled for smoking history [30].
Genome-wide association studies (GWAS) have implicated variants in several chromosomal regions associated with increased heritable lung cancer risk. These include the 5p15 locus, which includes the gene for the telomerase reverse transcriptase (TERT) [31], the 6p21 locus, which regulates G-protein signalling [32], and the 15q25–26 loci, which have been shown to increase nicotine dependence and lung cancer susceptibility [33]. Unlike in other common cancers, no specific germline genetic mutations (like BRCA1/2) or predisposing syndromes (Lynch syndrome) have been identified for lung cancer. Nevertheless, consideration of family history and genetic risk may improve the efficacy of early screening programs, especially among those with comorbid risk factors like smoking [30].
Modifiable risk factors
Tobacco smoking
More than 80% of lung cancer cases in the Western world are attributable to cigarette smoking. Tobacco smoking is considered the leading preventable cause of death worldwide, primarily due to the increased risk of lung cancer (along with neoplasms of the bladder, colorectum, and more) [4].
Nicotine is the addictive ingredient in tobacco smoking that binds to nicotinic acetylcholine receptors in the brain, altering gene and receptor expression and neurotransmitter levels to foster dependence. The combustion of tobacco produces over 60 known carcinogens, including polycyclic aromatic hydrocarbons (PAH) and N-nitrosamines. These compounds induce DNA damage and mutations that increase the risk of carcinogenesis across organ systems decades after use.
Cigarette smoking in Western nations greatly increased during the world wars, when soldiers were provided cigarettes to relieve their anxieties. Young men returning from the war were the first to adopt cigarette smoking, but the practice soon spread to become commonplace among women, and adolescents. It was not until 1950 that major epidemiological studies established the connection with increased risk of lung cancer [34]. Efforts since the 1960s have reduced smoking prevalence among US adults from 42.4% in 1965 to 13.7% in 2018 [35]. Proportional reductions in smoking prevalence among women in Western countries have lagged behind that of men, contributing to the disparately growing rates of lung cancer among women [4, 22]. Developing nations such as China, India, and Russia are projected to contribute to the growing global disease burden of lung cancer due to the continued high prevalence of tobacco smoking [4], with 65% of Chinese men smoking by their mid-20s [7].
In the US, tobacco smoking is currently associated with lower socioeconomic status and racial and gender-identity minorities. These populations are also associated with poorer access to nicotine addiction resources, preventive healthcare, and aggressive cancer treatment and clinical trials [16, 17, 36, 37]. This may help explain why among the 6.8 million Americans currently eligible for lung cancer screening based on extensive smoking history, only 4% have received screening [38, 39]. Prevention efforts aimed at reducing disparities in tobacco smoking and healthcare access among underprivileged populations are considered the most effective means to reduce lung cancer incidence in the Western world.
Second-hand smoke exposure likewise has shown a dose-dependent relationship with lung cancer risk. Certain carcinogens in second-hand smoke are inhaled in higher concentrations than by the smoker due to the filters on the user end of cigarettes; for instance, exposure to benzopyrene is 4 times greater [40]. Nonsmoking spouses of smokers are known to have a 20–30% increased risk of lung cancer. Exposure by pregnant women or children is associated with chronic conditions such as asthma, sudden infant death syndrome, and developmental delays [41–43].
Electronic cigarettes have risen in popularity in recent years, as a means of consuming nicotine or simulating smoking tobacco smoking without carcinogenic combustion products. However, studies suggest inhaled aerosol participles from e-cigarettes do contain polycyclic aromatic hydrocarbons, nitrosamines, and trace metals in varying concentrations, and long-term data on cancer risk are not yet available [44]. Due to the ingestion of higher concentrations of nicotine, e-cigarettes may be used for nicotine addiction, but are also highly addictive. While the CDC currently recommends that e-cigarettes may be used as a later-line option for overcoming cigarette-addiction, they are not considered safe for young adults and non-smokers due to increased case of E-cigarette or Vaping Use-Associated Lung Injury (EVALI). A 900% increase in e-cigarette usage among high school students in the US was reported between 2011 and 2015, with over 2 million middle and high school students reporting use [45].
Heated tobacco products (HTP) use battery power to heat tobacco to lower temperatures than traditional cigarettes, althoughbut they attain higher temperatures than e-cigarettes. HTPs were first introduced in 1988 as an alternative to traditional tobacco products. HTPs are considered safer than cigarettes but less safe than e-cigarettes in terms of carcinogenic risk [46].
Cannabis smoking
Cannabis, or marijuana, has been legalized for recreational use in 11 states and medical use in 33 states in the US. Reported marijuana use has increased in the US by 4% from 2002 to 2014, particularly among those with low income, though many surveys are considered underestimates due to the long and continued history of marijuana’s illegality [47]. Likewise, the effects of marijuana smoking on lung health have gone largely unstudied because of the drug’s illegal status.
The combustion of marijuana is known to produce carcinogenic substances, with levels of some, such as tar and polyaromatic hydrocarbons, higher than those in tobacco. Marijuana consumption has been shown to induce premalignant histological changes in bronchial epithelium similar to those with tobacco smoking. Case-control studies from North Africa found a 2.4-fold increased risk of lung cancer among men who regularly smoked marijuana, after adjustment for tobacco smoke and occupational exposure [48]. A case-control study from Europe confirmed these findings [49]. Large epidemiological studies have not found a strong association, though these results are stymied by likely underreporting of consumption [50]. Marijuana is considered less addictive than nicotine, and average daily consumption among marijuana users is far less than that of tobacco users [50]. Legalization and funding of marijuana research is likely to facilitate a better understanding of the risks and medicinal applications of cannabis.
Asbestos
Occupational exposure to carcinogens is estimated to account for 5–10% of global lung cancer cases, of which, asbestos is the most common contributor [22]. Asbestos is a naturally occurring mineral used in construction due to its flame-retardant properties. Asbestos is known to deposit fibres in the lungs and has been associated with varied lung pathologies including pneumoconiosis, bronchogenic lung cancer, and mesothelioma, a rare malignancy of the lung pleura [51–53]. The rate of lung cancer among non-smokers in a North American cohort of insulators (who regularly worked with asbestos) was increased 3.5 fold. Asbestos fibres are also known to trap tobacco particulates, explaining the synergistic effect of asbestos with tobacco smoking on lung cancer, with a cohort study reporting a 14.4-fold increased risk [54]. While highly regulated in the Western world, asbestos consumption is increasing in developing nations such as Asia and Latin America [55].
Radon
Radon is a gas naturally produced from the decay of uranium in the ground, which has mutagenic properties. Time spent below the ground, such as in basements or mines, especially in geographic regions with high Uranium concentrations, is associated with radon exposure. Underground workers mining for metals or uranium are known to have markedly increased risk of squamous cell carcinoma of the lungs and other organs [56–58]. Residential radon exposure is the second greatest risk factor for lung cancer in the Western world, accounting for an estimated 10% of cases [59]. Up to 30% of lung cancer cases in non-smokers are associated with radon [59]. Underground and residential radon exposure is regulated in the US, and systems to circulate air are recommended for all underground dwellings in high-risk locations, which includes much of the Northeast US. Radon exposure has a synergetic effect on lung cancer risk with tobacco smoking [59].
Air pollution
There are risk factors for lung cancer related to air quality: carcinogens from the combustion of fossil fuels, and particular matter suspended in the air. While atmospheric levels of carcinogens such as PAH are inhaled by all residents of industrial regions, workers with prolonged occupation exposure are at the highest risk. Truckers, who are chronically exposed to vehicular fumes on the road, have a 50% increased risk of lung cancer [60]. Similarly, many industrial workers are at heightened risk, especially in developing nations where safety standards are not as rigorously developed and enforced [60]. A study of large cities in the US found a 40% increased risk of lung cancer among the 6 cities with the greatest levels of particulate matter in the air. The association with cancer was greatest among nonsmokers. Particulate matter is designated as a group I carcinogen by the International Agency of Research on Cancer (IARC) and is highly regulated in vehicular and industrial production by the US Environmental Protection Agency [61].
Indoor air pollution from combustion products is particularly a concern in developing nations and rural communities, where wood and charcoal are commonly used for cooking and heating. Studies have found that ventilation of such cooking areas can reduce lung cancer risk by up to 50% [61].
Arsenic
Arsenic is a heavy metal classified as a group 1 carcinogen by the IARC. The primary source of exposure is leakage of inorganic aArsenic into groundwater; many agricultural and industrial laborers are exposed [62].
According to the IACR, aArsenic has been implicated in cancers of the skin, lungs, bladder, prostate, kidneys, and liver in humans [63, 64]. Arsenic has also been implicated in the development of vascular disease, including stroke and ischaemic heart disease [65]. Recent studies have also suggested diabetic, neurological, and reproductive sequelae [66]. People living in regions of Chile exposed to dramatically increased aArsenic levels in their drinking water in the 1950s–1970s demonstrated a 3.6-fold increased risk of lung cancer mortality over the subsequent 50 years [64].
Infection
Inflammation and cellular damage during respiratory infection have been implicated in lung carcinogenesis. Tuberculosis (TB), which is the leading infectious cause of death globally, conferred an odds ratio of lung cancer development of 1.76 according to a meta-analysis [67]. HIV also increases the risk of lung cancer by up to 2.5 times, irrespective of smoking status. In fact, with the development of anti-retroviral therapies that largely protect against AIDS-defining infection, lung cancer has become the leading cause of death among HIV patients in the US [68–70]. The most likely pathogenic mechanism is immunosuppression and impaired cancer surveillance. Organ transplant recipients on immunosuppressive therapies have similarly elevated rates of lung cancer. Patients with HIV are more likely to be minorities, low socioeconomic status, and smokers. The intersectionality of poor healthcare access and synergistic disease comorbidities may help explain why HIV patients also have lower rates of lung cancer survival than the general population [68–70]. The long-term sequelae of pulmonary inflammation, cytokine release storm, and acute respiratory distress seen in COVID-19 patients may include increased risk of lung cancer decades down the line [71].
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) primarily occurs due to smoking. COPD-related inflammation and scarring is independently associated with an increased risk of developing lung cancer. Mechanisms by which COPD can increase risk of lung cancer include increasing oxidative resulting in deoxyribonucleic acid (DNA) damage, prolonged exposure to inflammatory cytokines, suppression of DNA repair mechanisms, and increased cellular proliferation [72]. COPD is the most common independent risk factor, other than smoking, for lung cancer, increasing the risk of lung cancer by 6- to 13-fold [73, 74].
Conclusions
Lung cancer is the most common and deadly cancer worldwide, and its global disease burden is projected to rise with increasing tobacco-smoking rates. Lung cancer is more common among men, adults over 60 years old, African Americans, and those with a family history. Tobacco smoking accounts for over 80% of lung cancer cases and is the leading preventable cause of death worldwide. While smoking has decreased in the Western world, smoking is on the rise globally and in many developing nations like China. Further major contributors include radon, second- hand smoke, asbestos, air pollution, arsenic, and HIV and TB infection, while cannabis, e-cigarettes, and COVID-19 have been suggested to increase risk. Underprivileged populations are at higher risk of cigarette smoking, HIV and TB infection, poor air quality, lower access to healthcare, higher risk of lung cancer, and worsened lung cancer survival rates. Prevention efforts to curb the growing global burden of lung cancer should be targeted against risk factors such as smoking, occupational and environmental exposure, and HIV and Tb infection. Increasing access to screening, nicotine addiction programs, and aggressive/experimental lung cancer treatments among underprivileged populations can help reduce disparities. Further investigation into the impact of cannabis smoking, electronic cigarettes, and the long-term consequences of COVID-19 on lung cancer risk is required.








