Introduction
The necessity of accurate therapeutic decisions, adequate follow-up of response, and further disease management requires appropriate risk stratification, prediction, and prognosis in breast cancer (BC) patients. The administration of chemotherapy before surgery (neoadjuvant chemotherapy – NAC) is increasing, and it has become standard of care for advanced and high-risk human epidermal growth factor receptor 2-positive/HER2+/triple-negative BC. Recently, neoadjuvant therapy has been considered an optional strategy even in early BC patients, extending its benefits beyond downstaging the tumour, making it operable, but also providing prognostic information for further therapeutic decisions and better oncological outcomes [1]. The tumour, node, metastasis (TNM) staging system is still universally used in BC patients, but the application of the histological grade and contemporary biomarkers defines new pathologic insights that have changed the decision for treatment in almost 40% of the patients, giving clinicians better and more precise individual prognostication [2].
The most commonly used biomarkers in BC patients include oestrogen (ER), progesterone (PR), and human epidermal growth factor receptor 2 (HER2), and they are frequently enhanced by others having an independent prognostic value, such as proliferation index Ki-67, histological grade, or gene expression profiling, which is not always available in daily practice. Caspase-3 has recently been studied, mostly in combination with the Ki-67 proliferation index. It was found to have an additive negative predictive value on overall survival. Recent studies support the idea that disrupted apoptosis/proliferation balance contributes more to tumour progression or inhibition than either marker alone in BC patients [3]. The clinical prognostic group stratification is useful not only at baseline before any therapeutic intervention, but also after neoadjuvant therapy comparing with the initial clinical stage, thus providing an accurate assessment of response prior to the next therapy [4]. Still, there is an insufficient database to achieve accurate and precise staging for BC patients who are suitable to receive neoadjuvant systemic therapy before surgery. The prognostic staging often lacks complete information about the initial evaluation due to lab availability, inappropriate diagnostic method, or intra-tumour heterogeneity. More data are needed from a large population-based cohort [5].
The aim of this study was to assess how cleaved caspase-3 and Ki-67 index evaluated on diagnostic biopsy are related to response to NAC in the context of molecular subtype, post-treatment tumour T category, N category, and grade.
Material and methods
A retrospective analysis was carried out among 110 BC patients/cases (108 female and 2 male). Five patients were excluded from further analyses due to insufficient tissue samples. Based on the staging, the patients were divided as followed IIA 5 patients, IIB 48 patients, IIIA 24 patients, IIIB 23 patients, IIIC 3 patients, and IV 7 patients. Tissue samples from diagnostic biopsies and post-neoadjuvant therapy tissue samples were retrieved for further analysis. Tumours were typed, graded, staged, categorised (ypT and ypN category), and subdivided into surrogate immunohistochemically determined “molecular subtypes” according to the current World Health Organisation recommendations [6]. The characteristics of the studied cases are presented in Table 1.
Table 1
Characteristics of the studied cases
Treatment was according to contemporary guidelines [7]. In the studied population, some advanced-stage and heterogeneous tumours received neoadjuvant therapy and were included in the study. Neoadjuvant chemotherapy effect was estimated on resection specimens (on archival FFPE materials; in particular, representative haematoxylin and eosin-stained slides) using the Sataloff criteria [8]. Biopsies (tissue samples) taken prior to NAC and post-neoadjuvant therapy were analysed, and cleaved caspase-3 and Ki-67 index were investigated on tissue samples taken prior to NAC, using immunostaining according to protocols provided by the manufacturers (Ki-67 rabbit monoclonal antibody clone SP6/Biocare USA; caspase-3 rabbit polyclonal antibody/Biocare USA; visualisation system MACH1 Univ. HRP kit/Biocare USA). Staining was scored according to methods available in the literature [9, 10]. The expression of caspase-3 was evaluated according to Engels et al., and levels of > 0.5% were considered as increased [3]. Variables including Ki-67 levels on diagnostic biopsy, caspase-3 expression on diagnostic biopsy, and their relation to tumour grade, surrogate molecular subtype, ypT, ypN categories, and T and N categories according to the Sataloff criteria (on post-therapeutic resection specimens) were evaluated. Missing variables from cases (missing for reasons such as insufficient materials in the archival samples or other tissue-related reasons) were excluded from the individual analyses. Statistical analyses were performed using the ANOVA F, Kruskal-Wallis, and χ2 tests, and calculated using SPSS software. Values of p < 0.05 were considered significant. Permission for the current study was obtained from the Ethical Committee of the Medical University of Pleven.
Results
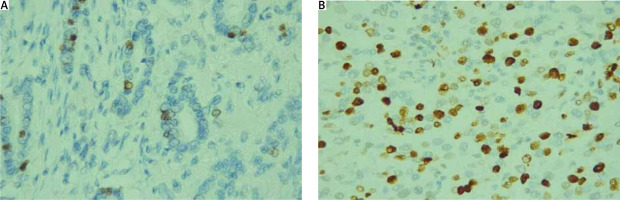
Tumour heterogeneity (morphologic/molecular) was observed in 12 of the cases. For the whole population, Ki-67 demonstrated a median value of 26% (ranging 8–94%). Examples of Ki-67 values of less than 14% and equal to or above 14% are demonstrated in Figure 1.
Fig. 1
A) Ki-67 values of less than 14%, Ki-67, 400×; B) Ki-67 values equal to or above 14% Ki-67, 400×
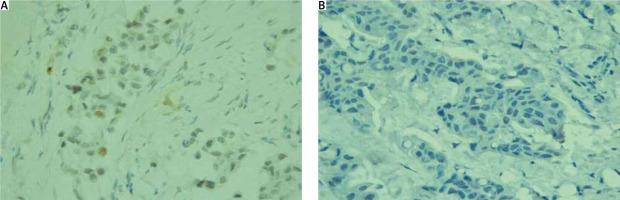
Caspase-3 was found positive in 17 cases and negative in 88 cases. Examples of positive and negative cases for caspase-3 immunostaining are presented in Figure 2.
In our study, the cases negative for caspase-3 demonstrated median values for Ki-67 = 25% (range 8–94%), compared to the cases positive for caspase-3, demonstrating values for Ki-67 = 35% (range 12–87%), with no significant difference between the 2 groups; K-W = 1.7631, p = 0.1842.
When compared to the studied prognostic factors and parameters, Ki-67 demonstrated the following relations: statistically significant difference was found between Ki-67 levels (on diagnostic biopsy) and degree of differentiation (G) K-W = 24.2932, p < 0.0001. As might be expected, low-grade (G1) tumours had the lowest Ki-67 index and G3 tumours had the highest Ki-67 index (Table 2). A statistically significant difference was found between Ki-67 levels (on diagnostic biopsy) and (surrogate) molecular subtype of the tumours (on diagnostic biopsies); K-W = 28.5439, p < 0.00000967538. Luminal A carcinomas were with the lowest Ki-67% index compared to the other categories (Table 2). A statistically significant difference was found between Ki-67 levels (on diagnostic biopsy) and the size and invasion of the primary tumour after NAC, radical surgery, and histopathological examination; K-W = 11.7944, p < 0.0377169. The tumours that demonstrated complete pathological response (referred to yT0 and yTis) were among the tumours with the highest Ki-67 in the studied group (Table 2). No statistically significant difference was found between Ki-67 levels (on diagnostic biopsy) and the extent of regional lymph node involvement after NAC, radical surgery, and histopathological examination; K-W = 4.19294, p > 0.241366 (Table 2). No statistically significant difference was found between Ki-67 levels (on diagnostic biopsy) and the effect of neoadjuvant therapy assessed according to the Sataloff classification category T; K-W = 3.2385, p = 0.0719229 (Table 2). No statistically significant difference was found between Ki-67 levels (on diagnostic biopsy), the effect of neoadjuvant therapy, and the Sataloff classification category N; K-W = 1.40937 p > 0.703338 (Table 2).
Table 2
Ki-67 and its relationship with the studied prognostic factors
When compared to the studied prognostic factors and parameters, caspase-3 demonstrated the following:
A tendency towards association was found between the expression of caspase-3 (on diagnostic biopsy) and the size and invasion of the primary tumour after neoadjuvant therapy and surgical removal, but the number of patients in the study was small, making the statistical significance unreliable; χ2 = 27.02, df = 5, p = 0. 0001 (Table 3).
A statistically significant association was found between the expression of caspase-3 (on diagnostic biopsy) and the effect of treatment with neoadjuvant therapy, evaluated according to the Sataloff classification; χ2 = 5.97, df = 1, p = 0.0145. More than half of the patients demonstrating complete pathologic response after neoadjuvant treatment were found to be caspase-3 positive on diagnostic biopsy, but only 1/8 of the non-responders were found to express caspase-3 on diagnostic biopsy (Table 4).
No statistically significant association was outlined between the expression of caspase-3 (on diagnostic biopsy) and the grade of differentiation; χ2 = 0.64, df = 2, p = 0.7251 (Table 5).
No statistically significant association was outlined between the expression of caspase-3 (on diagnostic biopsy) and YN status; χ2 = 2.7, df = 3, p = 0.4401 (Table 6).
No statistically significant association was outlined between the expression of caspase-3 (on diagnostic biopsy) and the Sataloff response category; N χ2 = 3.88, df = 3, p = 0.2746 (Table 7).
No statistically significant relation was found between the expression of caspase-3 (on diagnostic biopsy) and the molecular (surrogate) subtype of the tumour; χ2 = 6.98, df = 4, p = 0.1372 (Table 8). Additionally, the effect from neoadjuvant therapy in the context of proliferative Ki-67 index and the expression of caspase-3 on diagnostic biopsy was analysed separately for each therapeutic and molecular subtype.
Luminal tumours treated predominantly with epirubicin hydrochloride and cyclophosphamide demonstrated no statistically significant difference between Ki-67 levels (on diagnostic biopsy) and the effect of neoadjuvant therapy assessed according to the Sataloff classification category T; K-W = 0.0957, p = 0. 7570 (Table 9).
Luminal tumours treated predominantly with epirubicin hydrochloride and cyclophosphamide demonstrated no significant association between the expression of caspase-3 (on diagnostic biopsy) and the effect of treatment with neoadjuvant therapy, evaluated according to the Sataloff classification; χ2 = 0.19, df = 1, p = 0. 6592 (Table 10).
Luminal tumours treated predominantly with epirubicin hydrochloride and cyclophosphamide demonstrated no statistically significant difference between Ki-67 levels (on diagnostic biopsy) and the effect of neoadjuvant therapy, the Sataloff classification category N, K-W = 0.7656 p = 0.8577 (Table 9). Luminal tumours treated predominantly epirubicin hydrochloride and cyclophosphamide demonstrated no statistically significant association between the expression of caspase-3 (on diagnostic biopsy) and the Sataloff response category N; χ2 = 4.17, df = 3, p = 0.2434 (Table 11).
Human epidermal growth factor receptor 2-positive tumours treated with docetaxel, trastuzumab, and pertuzumab demonstrated no statistically significant difference between Ki-67 levels (on diagnostic biopsy) and the effect of neoadjuvant therapy assessed according to the Sataloff classification category T; F = 3.38, p = 0.0780 (Table 9). Human epidermal growth factor receptor 2-positive tumours treated with docetaxel, trastuzumab, and pertuzumab demonstrated a tendency towards association between the expression of caspase-3 (on diagnostic biopsy) and the effect of treatment with neoadjuvant therapy, evaluated according to the Sataloff classification; χ2 = 4.34, df = 1, p = 0.0372. Nearly half of the patients demonstrating complete pathologic response after neoadjuvant treatment were found to be caspase-3 positive on diagnostic biopsy. In contrast, less than 1/10 of the non-responders were found to express caspase-3 on diagnostic biopsy. The number of cases was small, and due to their distribution, no general conclusions can be made (Table 12).
Human epidermal growth factor receptor 2-positive tumours treated with docetaxel, trastuzumab, and pertuzumab demonstrated no statistically significant difference between Ki-67 levels (on diagnostic biopsy) and the effect of neoadjuvant therapy, assessed according to the Sataloff classification category N; F = 0.40 p = 0.7515 (Table 9).
Human epidermal growth factor receptor 2-positive tumours treated with docetaxel, trastuzumab, and pertuzumab demonstrated no statistically significant association between the expression of caspase-3 (on diagnostic biopsy) and the Sataloff response category N; χ2 = 1.97, df = 3, p = 0.5779 (Table 13).
Triple-negative tumours treated with paclitaxel and carboplatin demonstrated no statistically significant difference between Ki-67 levels (on diagnostic biopsy) and the effect of neoadjuvant therapy assessed according to the Sataloff classification category T; F = 0.01, p = 0.9278 (Table 9).
Triple-negative tumours treated with paclitaxel and carboplatin demonstrated no significant association between the expression of caspase-3 (on diagnostic biopsy) and the effect of treatment with neoadjuvant therapy, evaluated according to the Sataloff classification. No caspase-3 positive cases were found (Table 14).
Triple-negative tumours treated with paclitaxel and carboplatin demonstrated no statistically significant difference between Ki-67 levels (on diagnostic biopsy) and the effect of neoadjuvant therapy, evaluated according to the Sataloff classification category N; F = 0.83, p = 0.5582 (Table 9).
Triple-negative tumours treated with paclitaxel and carboplatin demonstrated no statistically significant association between the expression of caspase-3 (on diagnostic biopsy) and the Sataloff response category N; χ2 = 7,00, df = 3, p = 0.0.0719 (Table 15).
Table 3
Expression of caspase-3 (on diagnostic biopsy) and the size and invasion of the primary tumour after neoadjuvant therapy and surgical removal
| ypT | 0 | 1 | 2 | 3 | 4 | In situ |
|---|---|---|---|---|---|---|
| Caspase-3 negative, n (%) | 9 (8.57) | 17 (16.19) | 42 (40.00) | 8 (7.62) | 12 (11.43) | 0 (0.00) |
| Caspase-3 positive, n (%) | 3 (2.86) | 5 (4.76) | 5 (4.76) | 0 (0.00) | 0 (0.00) | 4 (3.81) |
Table 4
Expression of caspase-3 (on diagnostic biopsy) and the effect of treatment with neoadjuvant therapy, evaluated according to the Sataloff classification
| Sataloff T | A (complete response) | B, C, D (non-complete response and no response) |
|---|---|---|
| Caspase-3 negative, n (%) | 11 (12.36) | 63 (70.79) |
| Caspase-3 positive, n (%) | 7 (7.87) | 8 (8.99) |
Table 5
Caspase-3 (on diagnostic biopsy) and the grade of differentiation
| Grade | G1 | G2 | G3 |
|---|---|---|---|
| Caspase-3 negative, n (%) | 2 (1.94) | 40 (38.83) | 44 (42.72) |
| Caspase-3 positive, n (%) | 0 (0.00) | 7 (6.80) | 10 (9.71) |
Table 6
Expression of caspase-3 (on diagnostic biopsy) and yN status
| ypN | N0 | N1 | N2 | N3 |
|---|---|---|---|---|
| Caspase-3 negative, n (%) | 24 (23.53) | 27 (26.47) | 22 (21.57) | 12 (11.76) |
| Caspase-3 positive, n (%) | 8 (7.84) | 4 (3.92) | 4 (3.92) | 1 (0.98) |
Table 7
Caspase-3 (on diagnostic biopsy) and the Sataloff response category N
| Sataloff N | A | B | C | D |
|---|---|---|---|---|
| Caspase-3 negative, n (%) | 6 (7.06) | 17 (20.00) | 25 (29.41) | 22 (25.88) |
| Caspase-3 positive, n (%) | 2 (2.35) | 5 (5.88) | 7 (8.24) | 1 (1.18) |
Table 8
Caspase-3 (on diagnostic biopsy) and the molecular (surrogate) subtype of the tumour
Table 9
Ki-67 and its relation to treatment response in different tumour (therapy) groups
Table 10
Expression of caspase-3 (on diagnostic biopsy) and the effect of treatment with neoadjuvant therapy, evaluated according to the Sataloff classification
| Sataloff T | A (complete response) | B, C, D (non-complete response and no response) |
|---|---|---|
| Caspase-3 negative, n (%) | 1 (1.79) | 47 (83.93) |
| Caspase-3 positive, n (%) | 1 (1.79) | 7 (12.50) |
Table 11
Caspase-3 (on diagnostic biopsy) and the Sataloff response category N
| Sataloff N | A | B | C | D |
|---|---|---|---|---|
| Caspase-3 negative, n (%) | 1 (1.89) | 5 (9.43) | 22 (41.51) | 17 (32.08) |
| Caspase-3 positive, n (%) | 1 (1.89) | 2 (3.77) | 4 (7.55) | 1 (1.89) |
Table 12
Expression of caspase-3 (on diagnostic biopsy) and the effect of treatment with neoadjuvant therapy, evaluated according to the Sataloff classification
| Sataloff T | A (complete response) | B, C, D (non-complete response and no response) |
|---|---|---|
| Caspase-3 negative, n (%) | 8 (29.63) | 12 (44.44) |
| Caspase-3 positive, n (%) | 6 (22.22) | 1 (3.70) |
Table 13
Caspase-3 (on diagnostic biopsy) and the Sataloff response category N
| Sataloff N | A | B | C | D |
|---|---|---|---|---|
| Caspase-3 negative, n (%) | 3 (12.00) | 9 (36.00) | 3 (12.00) | 4 (16.00) |
| Caspase-3 positive, n (%) | 1 (4.00) | 3 (12.00) | 2 (8.00) | 0 (0.00) |
Discussion
Tumour size and lymph node status are independent prognostic factors but also additive, time dependent, and associated with a worsening prognosis [11]. The histological grade, assessed upon the degree of tubule formation, nuclear pleomorphism, and mitotic count, shows a very strong correlation with prognosis, but it is not identified as independent prognostic factor [12]. These 3 factors form the multifactorial Nottingham prognostic index were used to stratify patients for appropriate therapy. The molecular BC subtypes and proliferative and apoptotic index can be used as markers of efficacy evaluation before and after NAC, but they have limited independent significance, and their prognostic value has been inconsistent [4].
Ki-67 protein has long been studied as a proliferation marker expressed in dividing cells. Although loss of Ki-67 has been proven to have little or no impact on cell proliferation, it still plays a critical role in cancer development [13]. Mrouj et al. suggest that Ki-67 plays a role in cellular adaptation to the environment, thus favouring carcinogenesis and drug resistance, but also making tumour cells more sensitive to antitumour immune responses [14].
To date, a lot of studies have presented results showing that high Ki-67 expression in patients with early-stage BC is related to higher recurrence rate and poor overall survival [15, 16]. Other reports indicate that tumours with high Ki-67 expression respond more effectively to NAC [17] and are more likely to attain pathological complete response after NAC [4, 18]. High Ki-67 expression after NAC can be a poor prognostic marker for efficacy evaluation of NAC and subsequent therapy management [19]. Generally, Ki-67 is unlikely to predict the response to endocrine neoadjuvant therapy in hormone receptor-positive BC [20]. These observations are in accordance with the tendency observed in our study, where Ki-67 expression in patients with complete pathologic response was not significantly higher compared to the other groups. This may be a result of the relatively low number of patients, the antibody used, or tumour heterogeneity in about 10% of the studied population. It is also possible that the Ki-67 index might be predictive for a specific molecular subtype [21]. The therapy response in the N category in our study demonstrates that Ki-67 may be high in cases with either complete response or no response, but again it was not statistically significant, underlining the fact that neoadjuvant therapy response may not be predicted by Ki-67. This leads to the need for alternative methods of evaluation like dynamics in Ki-67 expression between pre- and post-NAC or follow-up in combination with other markers. Such a marker could be caspase-3, the most widely studied member of the cysteine protease family, which plays a central role in the induction of apoptosis in response to various stimuli, including chemotherapy. Apart from being an apoptotic executor, caspase-3 also takes part in other important cellular events like proliferation, differentiation, and migration, which may explain metastasis, and chemotherapy resistance in tumours [22].
Most studies confirm the relationship between the level of apoptosis and caspase-3 expression [23]. Liu et al. even suggest that cleaved caspase-3 is related to better chemotherapy response but worse prognosis [24]. Our study presents similar observations on caspase-3, suggesting that its expression may be related to response to neoadjuvant therapy, although it was not focused on overall survival.
A parallel increase in apoptosis and proliferation in progressive ductal epithelial lesions with proliferative activity significantly outweighing apoptotic activity leading to clonal expansion, and thus progression was observed by Bai et al. [25]. Such a predominance of apoptotic over proliferative activity in situ carcinomas/ DCIS/a possible explanation of their stable state, with delayed subsequent invasion [26, 27].
Cleaved caspase-3 and Ki-67 index on diagnostic biopsy are also found to be related to some of the post-treatment prognostic factors (tumour T stage, N stage, and grade), thus being complimentary to them in the prediction of treatment response and further disease management [28, 29].
Our results concerning the relationship between caspase-3 expression and prognostic factors in BC are in accordance with the data found in the literature, summarised in a meta-analysis of 3091 cases in 21 studies by Yang et al. up to 2017, in which an association of high levels of caspase-3 and progression and poor prognosis in patients with breast carcinoma was largely confirmed. The study did not prove an association between caspase-3 and TNM and histology grade, but a statistically significant relationship between PR and HER2 status was observed [30].
According to data from the current study, the response to therapy in luminal A, luminal B, and triple- negative tumours cannot be predicted based on Ki-67 expression or activated caspase-3 expression (evaluated on preoperative biopsy). In practice, a Ki-67-based follow-up to neoadjuvant endocrine therapy for stage 2 and stage 3 ER receptor-positive breast carcinomas is proposed [31]. It is still uncertain how applicable this method is to heterogeneous breast tumours.
Existing data demonstrate the prognostic value of caspase-3 for disease outcome in ER, PR, or HER2 subsets and non-basal-like subgroup [32]. Data are generally scarce and controversial, assessing expression of caspase-3 together with tumour size, age, histologic type, ER, progesterone receptor, HER2 status, lymph node status, prognosis, and overall survival [33].
Expression of Ki-67 and activated caspase-3 in the different therapeutic subgroups shows a tendency towards overexpression in HER2-positive tumours, where it is most often associated with a complete pathological response in the primary tumour [34].
According to our observations, some HER2-driven breast carcinomas preserve their apoptotic properties (presence of cleaved caspase-3), which makes them sensitive to anti-HER2 therapy (and good responders). In our opinion, Ki-67 shows a similar predictive potential for therapeutic response evaluation in HER2 tumours on target therapy.
In the context of tumour heterogeneity, the study of Ki-67 expression and activated caspase-3 on diagnostic biopsy samples is relevant in all types of tumours, and in practice they play a potentially significant role in the HER2-associated progression pathway. Systematic studies are required on these potential biomarkers in the context of neoadjuvant therapy effect prediction.
Conclusions
Caspase-3 expression was found to be significantly related to complete pathologic response to neoadjuvant treatment in the studied BC patients. Although there were noticeably increased Ki-67 values in patients who responded completely compared to patients with partial response or no response to neoadjuvant therapy, there was no statistical significance. The present study demonstrated that both markers have the potential to be used for prediction of the response to neoadjuvant therapy in BC. Further investigations are required to allow the possible introduction of the studied markers in routine diagnostic practice as predictors of neoadjuvant treatment response.