Introduction
Breast cancer is known as the most commonly diagnosed and the second leading cause of cancer-related mortality in women [1]. Similar to most solid tumors, breast cancer requires new blood vessel growth (i.e. neovascularization), and these new vessels not only help to meet the growing metabolic demands of the tumor by supplying additional nutrients but also provide potential routes for tumor dissemination and metastasis [2]. In breast cancer, tumor-induced angiogenesis is first evident at the pre-invasive stage of high-grade ductal carcinoma [3]. It is now evident that tumors have a very limited capacity to grow without vascular support. Therefore, the formation of blood vasculature is an obligatory step to sustain the influx of essential nutrients to the cancer cells [4].
Some proteases such as matrix metalloproteinase (MMPs) are considered to play important roles in immune responses, inflammatory reactions, and tissue remodeling [5, 6]. Recent studies confirmed that the expression of MMPs in tumor cells is closely correlated with their metastatic activity [7, 8]. The matrix metalloproteinase axis has several areas of overlap with the cytokine network, and inflammatory cytokines or growth factors can regulate the expression of MMPs. Cytokines and growth factors play an important role in promoting the activation of MMPs from the inactive zymogens to the active enzymes. In a recent study, several endothelial growth factors including vascular endothelial growth factor (VEGF) were demonstrated to promote neovascularization [9]. In fact, VEGF is a specific mitogen and survival factor for endothelial cells and a key promoter of physiological and pathological angiogenesis [10–12].
On the other hand, tamoxifen (TAM) is considered a gold standard in the treatment of estrogen receptor (ER)-positive breast cancer [13]. Estrogen receptors are one of the most important targets for control of carcinogenesis and inhibition of tumor cell growth. This drug is also effective as adjuvant therapy and considerably improves follow-up outcomes of surgery by reducing the risk of disease recurrence and death. The tamoxifen antitumor effect is first of all due to the ability to selectively block the ERs found in most breast cancer patients [13]. However, some side effects of TAM appear in breast cancer patients with long-term therapy [14].
Some plant-derived compounds such as flavonoids, phytoestrogens, and protease inhibitors were reported to be able to prevent one-third of cancers [15, 16] and inhibit tumor cell proliferation and new vessel formation in tumors without major side effects and significant toxicity to normal tissues [15, 17]. These natural compounds can provide protection against inflammatory diseases through the regulation of inflammatory pathways [17]. Some plants such as melon are a rich source of the protein trypsin inhibitor (TI), which has many biological functions including anti-oxidative, anti-inflammatory, and anticancer effects [15]. Melon seeds can be used as a source of nutrients, natural antioxidants, and bioactive compounds [18]. Recent studies showed that different component of melons have an anticancer role by affecting a variety of different mechanisms including cell proliferation, autophagy, level of insulin-like growth factor 1 receptor and its downstream signaling pathways. However, the main mechanism of these effects and also the effects of melon components on angiogenesis in breast cancer are unknown [19–22].
The present study aimed to investigate the effect of bioactive compounds of melon seed including TI and an extract of melon seed powder (EXT) on the expression of angiogenesis genes including MMP-2 and 9 and VEGF in vivo and in vitro on the MC4–L2 breast cancer cell line. We also assessed the changes in tumor tissue characteristics in the mouse model such as inflammation, necrosis, angiogenesis, cell proliferation, and tumor size.
Material and methods
Seed preparation of target plant
First, the seeds of the melon plant were prepared through washing them with water to remove any kind of contamination. The seeds were then dried indirectly using sunlight and the kernels were separated and crushed using a grinder. The resulting powder was used as a starting material to purify the target peptides by chromatography.
Preparation of affinity column with trypsin ligand and chromatography
After preparation of the specified seed powder, the chromatography method was applied as previously described elsewhere [23]. Our method differed only in the last step, in which the supernatant was loaded onto the column, then the column was washed with deionized water until the absorbance of fractions changed from 280 nm to zero. Three column volumes of deionized water with pH = 2.5 accustomed to wash sure proteins from the column (deionized water was adjusted to pH = 1.5 with 0.1 N HCl). After preparation of the specified seed powder, the chromatography method was done as described elsewhere.
Polyacrylamide gel electrophoresis
Polyacrylamide gel electrophoresis was performed in the presence of sodium dodecyl sulfate SDS-PAGE based on the Schagger and Von Jagow method [24] as previously explained [23].
Measurement of protein concentration
The final and quantitative protein concentrations were determined by the Bradford method as the standard procedure [25].
Assay of trypsin inhibitor activity
The activity of the TI from Cucumis melo (muskmelon) was determined by the residual trypsin activity following the method of Hajela et al. with slight modifications using N-α-benzoyl-DL-arginine-p-nitroanilide (BApNA) as the substrate and bovine trypsin as the standard enzyme. The reaction mixture containing 50 µl of TI (5 mg/ml), 50 µl of trypsin (1 mg in 5 ml of 0.05 M Tris-HCl, pH 8.0, containing 0.03 M CaCl2) and 100 µl of 0.05 M Tris-HCl (pH 8.0) containing 0.03 M CaCl2 was incubated at 37°C for 10 min in a shaking water bath. The residual activity was measured by adding 1 ml of 0.8 mM BApNA (7 mg dissolved in a minimum volume of dimethyl sulfoxide – DMSO, and adjusting its final volume to 20 ml with 0.05 M Tris-HCl, pH = 8.0, containing 0.03 M CaCl2) to the reaction mixture followed by incubation at 37ºC for 10 min in a shaking water bath. The reaction was stopped by adding 20 µl of 30% (v/v) glacial acetic acid. A blank and a trypsin control were run simultaneously. In the blank, acetic acid was added prior to the addition of BApNA and in the trypsin control, distilled water was added in place of the TI. The absorbance was recorded at 410 nm against the blank using a double beam UV-visible spectrophotometer (Model 2202, Systronics, India). An appropriate volume of the kidney bean extract, which was enough to give 40–60% inhibition of trypsin, was taken for the assay. One trypsin unit (TU) was defined as an increase of 0.01 absorbance units at 410 nm per 1.2 ml of the reaction mixture. Trypsin inhibitor activity was expressed as the number of trypsin units inhibited.
In vitro phase
Cell line and culture conditions
MC4–L2 mouse breast cancer cell lines (National Center for Genetic and Biological Resources of Iran, Tehran) were maintained and grown in 25 and 75 cm2 flasks (SPL, Pocheon, Korea) in DMEM: Ham’s F12 + 2 mM L-glutamine + 15 mM HEPES buffer, penicillin (100 µg/ml), streptomycin (100 µg/ml), and 10% (vol/vol) fetal bovine serum (FBS, Gibco BRL, Life Technologies, Grand Island, NY) in a 37°C incubator and 5% WEEK. Cells were monitored by a phase-contrast microscope until they reached appropriate confluence. Once the cells reached 90% confluency, the MC4–L2 cells was harvested with 0.25% trypsin – 0.02% ethylenediaminetetraacetic acid. Cell viability and numbers were determined by a hemocytometer and trypan blue exclusion. Cell viability was calculated to be greater than 98%.
Cell viability assay in vitro
Toxicity and cell proliferation were assessed using the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (MTT) Sigma test. First, to determine and set up the exact number of cells required to perform the desired test in a 96-’s pellet in 8 rows of 12 wells, different values of 5 * 103, 10 * 103, 15 * 103, 20 * 103, 25 * 103, 30 * 103, 50 * 103 and 100 * 103 of the MC4–L2 cell line were poured into 10% FBS-enriched DMEM-F12 medium to evaluate cell growth. After 24 hours, the cell growth rate was examined using a microscope and the number of 104 cells per well of the pellet had the best response, which was selected as the number of cells approved for the colorimetric test for assessing the metabolic activity of cells defined as MTT test. To perform the MTT test, 104 cells of MC4–L2 cell line were poured into each well of the 96-well culture plate and then 10% FBS enriched with 100 ml of DMEM-F12 culture medium per 100 ml was added. After 24 hours of incubation at 37°C with 5% CO2, different concentrations of TI (5, 10, 25, 50, 100, 200, 300, 400, 800, 1200 µg/ml), EXT (5, 10, 25, 50, 100, 200, 400, 800, 1200 µg/ml) and, TAM (0.01, 0.1, 1, 5, 10, 15, 20 µM) were added to each well and then 100 ml of the desired culture medium was added. The cells were incubated again for 48 hours and these steps were repeated 3 times for all concentrations. After 48 hours of incubation at 37°C with 5% CO2, the equivalent of 10 microliters of 3-(4.5–dimethylthiazole-2)-2,5-diphenyltetrazolium bromide MTT solution (Sigma) (0.5 mg/ml MTT powder in phosphate buffered saline – PBS), was added to each of the culture medium houses and incubated again for 4 hours at 37°C with 5% CO2 and then centrifuged at 3000 rpm for 10 minutes. To dissolve the Formazan crystal, the supernatant containing MMT was completely removed and 200 ml of DMSO was added to each well and kept at room temperature for 30 minutes to dissolve completely. The ELISA reader was read at 570 nm and 630 nm.
Examination of anti-angiogenesis effects of trypsin inhibitor, extract of melon seed powder, and TAM
According to MTT results and after preparation of the MC4–L2 cell line, 104 cells were poured into each well of a 96-well plate and placed in an incubator for 24 hours. Then we emptied the medium on the wells and 500 µl of fresh medium with 10% FBS was added to the wells. The control group received no treatment. Treatments groups were designed as BPS solution, 5 µM of TAM, 400 µg/ml of EXT, TI at concentrations of 200 and 300 µg/ml, and 300 µg/ml of TI + 5 µM of TAM. Then the plates were incubated at a CO2 incubator for 72 h. Five treatments were carried out for each dose. Finally, the anti-angiogenesis effects were examined using fluorescent staining and the reverse transcription polymerase chain reaction (RT-PCR) method.
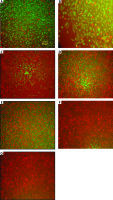
Fluorescent staining method and viability test
Phosphate-buffered saline 1X solution, fluorescein diacetate (FDA) and propidium iodide were used in a proportion of 1 ml, 10 µl, and 100 µl, respectively. Images were recorded using a microscope camera (Fig. 1).
Fig. 1
Evaluation of photomicrography by fluorescent staining method. Control (A), phosphate-buffered saline (B), tamoxifen (TAM) 5 µM (C), extract of melon seed powder 400 µg/ml (D), trypsin inhibitor (TI) 200 µg/ml (E), TI 300 µg/m (F), TAM 5 µM + TI 300 µg/ml groups indicated by inverted microscopy (G)
Green and red areas represent living and dead cells, respectively.
Animal phase
Experimental animals and tumor model
The Ethics Committee at Shiraz University of Medical Sciences approved the experiments (IR.SUMS.REC.1398.950). Five- to six-week-old normal female BALB/c inbred female mice were purchased from Pasteur Institute (Tehran, Iran). The mice were housed in an animal facility at a temperature of 22–24°C and 65% humidity. Trypsinized MC4–L2 cells were then harvested and washed to induce tumor formation in the mice. Their concentration was adjusted to 3.5 × 106 cells/100 µl with PBS at less than 98% viability. Prepared cells were injected subcutaneously into the right upper thigh of each mouse. Approximately 7–10 days after injection of the cancer cells, the tumors were palpated in the injected areas. The BALB/c inbred mice were randomly divided into six groups of five mice per group: controlled breast cancer mice without any treatment (normal control group), breast cancer mice treated with either 300 µg/ml or 600 µg/ml of TI, breast cancer mice treated with 800 µg/ml of EXT, the breast cancer mouse group received 10 µM TAM and the last group consisted of breast cancer mice that received combination therapy of 600 µg/ml of TI + 10 µM TAM. All mentioned doses were injected subcutaneously into the tumor area every day. According to our pilot study, the treatment period duration was 14 days. Finally, the mice were first anesthetized and then killed and their tumor tissue was extracted and stored in 10% formalin. Animal tests were begun within 24 hours (supplementary file).
Histological assessments
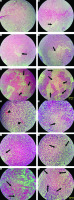
Tissue passage steps, preparation of paraffin blocks, and preparation of 5-micron sections were performed. Hematoxylin and eosin stain staining was performed for histological assessments using undiluted Mayer’s hematoxylin (Merck, Darmstadt, Germany) and 0.5% eosin (Merck). Evaluations were performed with a light microscope (Olympus cx31) for the intensity and scoring of inflammation (–, –/+, and +/+), necrosis (%), and peripheral vessels as angiogenesis (+, ++, +++) (Fig. 2).
Fig. 2
Histopathology evaluation of breast tumor tissue in experimental groups by hematoxylin and eosin stain method (A–F, 100×) Control: breast cancer control group (A, G), tamoxifen (TAM): breast cancer TAM 10 µM group (B, H), extract of melon seed powder (EXT) 800: breast cancer extract 800 µg/ml group (C, I), trypsin inhibitor (TI) 300: breast cancer TI 300 µg/ml group (D, J), TI 600: breast cancer TI 600 µg/ml group (E, K), TAM + TI 600: breast cancer TAM 10 µM + TI 600 µg/ml (F, L)
The white areas represent the shattered nuclei. The arrow sign indicates necrotic areas and the arrowhead indicates blood vessels. In the control group, tumor cells continued to grow and multiply and angiogenesis is ongoing. A significant decrease in the mean score of angiogenesis was observed in the groups receiving TI 600 and TAM+ TI 600 compared to the control group. The results showed a significant increase in the percentage of tumor tissue necrosis in the groups receiving TAM, EXT 800, TI 300, TI 600, and TAM + TI 600 compared to the control group. The results showed a significant increase in the mean score of inflammation in all groups compared to the control group. A–F 100×, G–L 400× indicate magnification
Molecular phase
RNA extraction and cDNA synthesis
Total RNA was extracted from MC4–L2 cell line-treated and tumor tissues of mice using TRIZOL reagent (Gene All, South Korea), according to the manufacturer’s instructions. RNA concentrations were determined using the NanoDrop spectrophotometer (Thermo Scientific, Germany). The quality of extracted RNA was assessed by 1% agarose gel electrophoresis. After RNA extraction, the complementary DNA (cDNAs) was synthesized using a cDNA synthesis kit (EURx, Poland), according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction
Real-time polymerase chain reaction was used to determine the expression levels of MMP-2, MMP-9, and VEGF genes in the MC4–L2 cell line and tumor tissue of mice, with the aim of quantification of the expression of angiogenic and anti-angiogenic factors.
Design of primers used for RT-PCR was done using Allele ID 6 software and primers are listed in Table 1. Subsequently, the primer specificity was confirmed by Primer- BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast) and In-Silico PCR (https://genome.ucsc.edu/cgi-bin/hgPcr. The human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was considered as the housekeeping gene (internal control).
Table 1
Nucleotide sequences of primers used for gene expression analysis by real-time polymerase chain reaction
The real-time PCR reactions were performed in duplicate using the SYBR Green PCR master kit (EURx, Poland) in a real-time PCR instrument (Applied Biosystems, USA). The expression of the gene of interest (MMP-2, MMP-9, and VEGF) at the transcript level were normalized to the GAPDH gene expression, and the standard deviations were calculated. Relative real-time PCR was performed in duplicate, and each experiment was repeated twice. The program for thermocycling was as follows: 1 cycle at 95°C for 2 min, followed by 40 cycles at 95°C for 30 seconds, and then 1 cycle at 65°C for 20 seconds. Finally, relative quantification of gene expression was carried out using the comparative Ct method [26].
Statistical analyses
Histopathological factors and tumor characteristics were assessed using the Kruskal-Wallis test. The Livak method (2–ΔΔCT) was used to compare the differences in expression levels of genes and the fold changes in treated and control groups. Due to the control group being compared with itself, the average of ΔΔCT became zero, and (2)0 = 1 was used as the control setup. One-way ANOVA was used for other parameters with the least significant difference test used as the post-hoc test. Statistical analyses were performed using SPSS software (version 22.0; IBM Corporation, Armonk, NY, USA). The results were considered to be significant when the p-values were < 0.05.
Results
Protein purification and electrophoresis
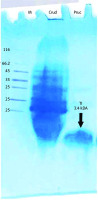
Electrophoresis analysis of purified protein by the Hejela method [27] identified a single band with a molecular mass of 3.4 kDa (Fig. 3, Table 2).
Anti-proliferative effect of trypsin inhibitor, extract of melon seed powder, and tamoxifen
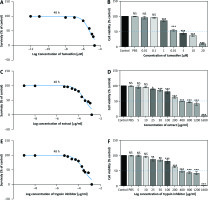
The MTT results showed that TI and EXT in doses of 5–1600 µg/ml and TAM in doses of 0.01–20 µM induced a significant reduction in the proliferation of MC4–L2 breast cancer cells which was dose-dependent with a half-maximal inhibitory concentration value of about 300 µg/ml, 400 µg/ml, and 5 µM respectively (Fig. 4). All concentrations were tested in triplicate, and results were accumulated from three sets of tests.
Fig. 4
2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide assay of tamoxifen, extract of melon seed powder, and trypsin inhibitor protein on MC4–L2 cell line after 48 h. TAM, extract of melon seed powder and trypsin inhibitor inhibited the growth of MC4–L2 cell line at 0.01–20 µM, 5–1600 µg/ml respectively (A, C, E), TAM, extract of melon seed powder, and TI have no cytotoxic effect on MC4–L2 cell line at 0.01–20 µM, 5–1600 µg/ml, respectively (B, D, F)
Data are presented as mean ±SD. All concentrations were tested in triplicate.
Effect of trypsin inhibitor, extract of melon seed powder, and tamoxifen on MMP-2, MMP-9 and vascular endothelial growth factor secretion in vitro and in vivo
In vitro results on the MC4–L2 cell line showed significantly lower MMP-2 gene expression in the groups receiving TI300 (99.9%, p < 0.05) and TAM + TI300 (99.92%, p < 0.01) compared to the control group and PBS. There was no significant difference between the other groups (Fig. 5 A). In addition, the expression of MMP-2 in the breast tumor tissue was significantly lower in all groups, i.e. TAM, EXT800, TI300, TI600, and TAM + TI600 (98%, 97.12%, 97.93%, 99.94%, 99.99% respectively, p < 0.001) groups, compared to the control group. There was also significantly lower expression of the MMP-2 transcript gene in the TAM + TI600 (99.76%, p < 0.05) group compared to the EXT800 group. There was no significant difference between the other groups (Fig. 6 A).
Fig. 5
In vitro evaluation of MMP-2, MMP-9, and vascular endothelial growth factor secretion by reverse transcription polymerase chain reaction method on MC4–L2 cell line (A–C)
Control: Medium culture + MC4–L2 cell line as control group; BPS: Medium culture + MC4–L2 cell line + phosphate-buffered saline (PBS) solution; tamoxifen (TAM): medium culture + MC4–L2 cell line + TAM 5 µM group; extract of melon seed powder 400: medium culture + MC4–L2 cell line + extract 400 µg/ml group; trypsin inhibitor (TI) 200: medium culture + MC4–L2 cell line + TI 200 µg/ml group; TI 300: medium culture + MC4–L2 cell line + TI 300 µg/ml group; TAM + TI 600: medium culture + MC4–L2 cell line + TAM 5 µM + TI 300 µg/ml. A) *, **: TI 300 and TAM + TI 300 vs. Con at p < 0.05 and p < 0.01, respectively; θ&θθ: TI 300 and TAM + TI 300 vs. PBS. B) **: TAM + TI 300 vs. Con at p < 0.01; θ&θθ: TI 300 and TAM + TI 300 vs. PBS. D) *, **: TI 300 and TAM + TI 300 vs. Con at p < 0.05 and p < 0.01, respectively; θ: TAM + TI 300 vs. PBS. Each data point is presented as mean ±SD.
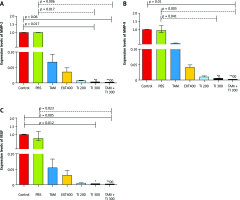
Fig. 6
In vivo evaluation of MMP-2, MMP-9, and vascular endothelial growth factor secretion by reverse transcription polymerase chain reaction method in breast cancer mice (A–C)
Control: breast cancer control group; tamoxifen (TAM): breast cancer TAM 10 µM group; extract of melon seed powder (EXT) 800: breast cancer extract 800 µg/ml group; trypsin inhibitor (TI) 300: breast cancer TI 300 µg/ml group; TI 600: breast cancer TI 600 µg/ml group; TAM + TI 600: breast cancer TAM 10 µM + TI 600 µg/ml A) ***: All treated groups vs. Con at p < 0.001; †: TAM + TI 600 vs. EXT 800. B) ***: All treated groups vs. Con at p < 0.001; †, ††: TI 600 and TAM + TI 600 vs. EXT 800. C) ***: All treated groups vs. Con at p < 0.001. Each data point is presented as mean ±SD.
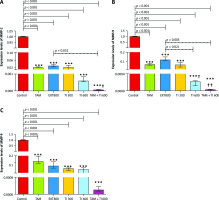
On the other hand, there was significantly lower expression of the MMP-9 transcript gene of the MC4–L2 cell line in the TAM + TI300 (99.93%, p < 0.01) group compared to the control group. Also, there was significantly lower expression of the MMP-9 transcript gene in the TI300 (99.65%, p < 0.05) and TAM + TI300 (99.93%, p < 0.01) groups compared to the PBS group. There was no significant difference between the other groups (Fig. 5 B). In addition, the expression of the MMP-9 transcript gene of breast tumor tissue was significantly lower in all study groups, i.e. TAM, EXT800, TI300, TI600, and TAM + TI600 (99.36%, 98.86%, 99.40%, 99.98%, ~100% respectively, p < 0.001) groups, compared to the control group. There was also significantly lower expression of the MMP-9 transcript gene in the TI600 (98.24%, p < 0.05) and TAM + TI600 (99.74%, p < 0.01) groups compared to the EXT800 group. There was no significant difference between the other groups (Fig. 6 B).
Regarding the VEGF transcript gene, the results of the study indicated significantly lower expression of the VEGF transcript gene in both TI300 (99.87%, p-value < 0.05) and TAM + TI300 (99.97%, p-value < 0.01) groups compared to the control group in the MC4–L2 cell line. Also, the expression of the VEGF transcript gene in the groups receiving TAM + TI300 (99.96%, p-value < 0.05) was significantly lower compared to the PBS group. There was no significant difference between the other groups (Fig. 5 C). The results showed significantly lower expression of the VEGF transcript gene at the tumor tissue level in all treated groups, i.e. TAM, EXT800, TI300, TI600, and TAM + TI600 (98.83%, 99.3%, 99.57%, 99.52%, 99.98% respectively, p-value < 0.001) groups, compared to the control group. There was no significant difference between the other groups (Fig. 6 C).
Effect of trypsin inhibitor, extract of melon seed powder, and tamoxifen on angiogenesis, inflammation, and tissue necrosis
A significantly lower mean score of angiogenesis was observed in the groups receiving TI600 and TAM + TI600 compared to the control group (p = 0.018 and p = 0.009, respectively). There was no significant difference between the other groups (Fig. 7 A).
Fig. 7
Evaluation of angiogenesis, necrosis, and inflammation of breast tumor tissue (A–C)
Control: breast cancer control group; tamoxifen (TAM): breast cancer TAM 10 µmoll group; extract of melon seed powder (EXT) 800: breast cancer extract 800 µg/ml group; trypsin inhibitor (TI) 300: breast cancer TI 300 µg/ml group; TI 600: breast cancer TI 600 µg/ml group; TAM + TI 600: breast cancer TAM 10 µM + TI 600 µg/ml. A) angiogenesis: *, **: TI 600 and TAM + TI 600 vs. Con at p < 0.05 and p < 0.01, respectively; B) necrosis: *, **, ***: TAM, EXT 800, TI 300, TI 600 and TAM + TI 600 vs. Con at p < 0.05, p < 0.01 and p < 0.001, respectively; †: TAM + TI600 vs. TAM and EXT 800. C) inflammation: ***: All treated groups vs. Con at p < 0.001. Each data point is presented as mean ±SD.
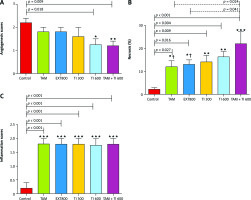
The results showed a significantly higher percentage of tumor tissue necrosis in the groups receiving TAM, EXT800, TI300, TI600 and TAM + TI600 compared to the control group (p < 0.027, p = 0.016, p = 0.009, p = 0.004, and p < 0.001, respectively). Also, a significantly higher percentage was observed in the TAM + TI600 group compared to the TAM and EXT800 groups (p = 0.024 and p = 0.041, respectively). There was no significant difference between the other groups (Fig. 7 B).
The results showed a significantly higher mean score of inflammation in all groups compared to the control group (p < 0.001). There was no significant difference between the other groups (Fig. 7 C).
Effect of trypsin inhibitor, extract of melon seed powder, and tamoxifen on body weight and breast tumor tissue characteristics
The results indicated no significant difference in body weight between groups over time (Fig. 8 A). There was a significant decrease in the mean tumor volume in all treated groups, TAM (131.94 ±6.83, p < 0.001), EXT800 (244.97 ±90.14, p < 0.01), TI300 (205.08 ±54.79, p < 0.001), TI600 (81.05 ±13.73, p < 0.001), and TAM + TI600 (161.13 ±36.47, p < 0.001) compared to the control group (558.35 ±26.68). No significant difference was observed between the other groups on other days (Fig. 8 B).
Fig. 8
Evaluation of body weight and tumor characteristics in experimental groups (A–E)
Control: breast cancer control group; tamoxifen (TAM): breast cancer TAM 10 µM group; extract of melon seed powder (EXT) 800: breast cancer extract 800 µg/ml group; trypsin inhibitor (TI) 300: breast cancer TI 300 µg/ml group; TI 600: breast cancer TI 600 µg/ml group; TAM + TI 600: breast cancer TAM 10 µM + TI 600 µg/ml. A) *: All treated groups vs. Con at p < 0.05; B) **, ***: TAM, EXT 800, TI 300, TI 600 and TAM + TI 600 groups vs. Con at p < 0.01 and p < 0.001, respectively; C) *, **, ***: TAM, TI 300, TI 600 and TAM + TI 600 vs. Con at p < 0.05, p < 0.01 and p < 0.001, respectively; D) *: TAM and TI 600 vs. Con at p < 0.05; E) *, **, ***: TAM, TI 300, TI 600 and TAM + TI 600 vs. Con at p < 0.05, p < 0.01 and p < 0.001, respectively. Data are presented as mean ±SD.

The results showed a significantly lower mean tumor width in the treated groups TAM (5.88 ±0.25, p < 0.05), TI300 (5.62 ±0.49, p < 0.01), TI600 (5.22 ±0.48, p < 0.01), and TAM + TI600 (4.82 ±0.32, p < 0.001) (i.e. all except the group receiving EXT800), compared to the control group (9.20 ±0.36). There was no significant difference between other groups on different days (Fig. 6 C).
A significantly lower mean tumor length was observed in the groups receiving TI600 (4.72 ±0.28, p < 0.01) and TAM (5.20 ±0.46, p < 0.01) compared to the control group (9.08 ±0.33) in the second week of treatment. No significant difference was observed between other groups on different days (Fig. 8 D).
There was a significantly lower mean tumor depth in the second week in all treated groups, i.e. TAM (3.78 ±0.26, p < 0.05), EXT800 (3.16 ±0.45, p < 0.01), TI300 (3.44 ±0.23, p < 0.01), TI600 (2.57 ±0.34, p < 0.01), and TAM + TI600 (3.84 ±0.76, p < 0.05), compared to the control group (5.76 ±0.21). No significant difference was observed between the other groups on other days (Fig. 8 E).
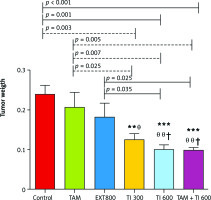
The results also show a significantly lower mean tumor weight in the groups receiving TI300 (0.124 ±0.015, p < 0.01), TI600 (0.099 ±0.013, p < 0.01), and TAM + TI600 (0.099 ±0.005, p < 0.01) compared to the control group (0.240 ±.0.022). Also, a significantly lower tumor weight was observed in the groups receiving TI300 (0.124 ±0.015, p < 0.05), TI600 (0.099 ±0.013, p < 0.01), and TAM + TI600 (0.099 ±0.005, p < 0.01) compared to the TAM group (0.208 ±0.036). The groups receiving TI600 (0.099 ±0.013, p < 0.05) and TAM + TI600 (0.099 ±0.005, p < 0.05) showed a significantly lower tumor weight compared to the EXT800 group. There was no significant difference between the other groups (Fig. 9).
Fig. 9
Evaluation of tumor weight in experimental groups at the end of the interventions
Control: breast cancer control group; tamoxifen (TAM): breast cancer TAM 10 µM group; extract of melon seed powder (EXT) 800: breast cancer extract 800 µg/ml group; trypsin inhibitor (TI) 300: breast cancer TI 300 µg/ml group; TI 600: breast cancer TI 600 µg/ml group; TAM + TI600: breast cancer TAM 10 µM + TI 600 µg/ml. **: All treated groups vs. Con; θ, θ θ: TI300, TI600, and TAM 10µM + TI 600 groups vs. TAM; †: TI600 and TAM + TI600 groups vs. EXT800. Data are presented as mean ±SD.
Discussion
This study was the first to explore the anti-angiogenic potential of Cucumis melo TI, EXT, and combination therapy of TI and TAM in both in vitro and in vivo situations in the MC4–L2 breast cancer cell line and tumor tissue in mice. The results indicated that TI, EXT, TAM, and adjuvant treatment of TI + TAM resulted in a reduction in expression of MMP-2, MMP-9, and VEGF (Fig. 10) and necrosis. Melon is one of the medicinal plants that have various antitumor and antioxidant compounds. It is also one of the drug supplements that can be effective in the treatment of cancer with various mechanisms. This plant has anti-inflammatory, anti-proliferative, anti-tumor, antioxidant effects and can regulate the immune system. One of the most important components of this plant’s seed is protease inhibitors, which are classified into cysteine protease inhibitors, serine protease inhibitors, and metallocarboxy protease inhibitors [23, 28, 29].
Fig. 10
Measurement of tumor dimensions at the end of the interventions. Control: breast cancer control group (A, G) Tamoxifen (TAM): breast cancer TAM 10 µM group (B, H), extract of melon seed powder (EXT) 800: breast cancer EXT 800 µg/ml group (C, I), trypsin inhibitor (TI) 300: breast cancer TI 300 µg/ml group (D, J), TI 600: breast cancer TI 600 µg/ml group (E, K), TAM + TI 600: breast cancer TAM 10 µM + TI 600 µg/ml (F, L)
There was a significant decrease in the mean tumor volume in all treated groups compared to the control group at the end of the interventions. But this reduction was greater in the TAM + TI600 group than the other groups.

Previous studies have reported that protease inhibitor consumption can decrease the risk of cancer development by inhibition of angiogenesis [30]. Since the formation of new blood vessels is one of the critical stages of tumor growth, angiogenesis inhibition can be one of the most important approaches in cancer prevention [31].
Our results identified that these treatments had a beneficial effect on angiogenesis inhibition by reduction or inhibition of MMP-2, MMP-9, and VEGF transcript gene expression. These treatments could also improve the breast tumor characteristics and had a beneficial effect on the increase of tumor necrosis and reduction of peripheral vessels in comparison to the control group.
One study reported similar results of TI from Cucumis melo on the expression of angiogenesis-related genes such as VEGF, MMP-2 and 9 in breast cancer cells [23]. In another study, a similar effect of TI protein extracted from soybean on inhibition of angiogenesis was observed [32].
According to previous studies, plant-derived compounds specifically inhibited tumor cell proliferation and new vessel formation in tumors without significant toxicity to normal tissues or major side effects [17, 33]. Also, several studies have already reported the cytotoxic, antioxidant/anti-inflammatory, and immunomodulatory effects of this fruit extract [34].
Melon seeds were reported to be a good source of natural active components and have antioxidant properties [35–37]. In fact, plant seeds contain two major families of protease inhibitors, the Kunitz and Bowman-Birk inhibitors, which have been studied previously as anticancer agents [38–40]. Some protease inhibitors from other sources have also been studied in relation to cancer development. Since the most common cause of cancer death in humans is angiogenesis-mediated metastasis of the primary tumor, angiogenesis modulation can be a promising approach to treat cancer [41, 42].
Angiogenesis is a multistep process involving degradation of basement membrane and extracellular matrix components, proliferation, migration, and tubulogenesis of endothelial cells, and finally maturation of the neovasculature. In the present study, TI and melon seed extract inhibited expression of VEGF, MMP-2 and 9 from MC4–L2 cells, and breast tumor tissue in mice. Vascular endothelial growth factor, as the most important antiangiogenic factor, plays a key role during the angiogenesis process, which involves induction of endothelial cell proliferation, migration, and also MMP secretion [43]. According to these results, TI and EXT’s suppressive effect on the expression of VEGF, MMP-2 and -9, which affects other important events during angiogenesis, might be considered as one of the mechanisms of their anti-angiogenic activity. However, more studies are required to exactly determine the anti-angiogenesis mechanisms of TI and EXT.
Our results also showed that all treatments led to an increase in necrosis and inflammation in tumor tissue and a significant decrease in peripheral vessels that led to a reduction of angiogenesis in comparison to the control group. The results also demonstrated that the TAM + TI600 group appeared to be more beneficial than the other treatments and the control group. Similar results were reported in some studies about TI and TAM’s beneficial effects on necrosis, inflammation, and angiogenesis [23, 32, 44].
Previous studies have indicated that inflammation increased vascular permeability, in which the leukocytes migrate into the injured tissues. Inflammatory mediators such as TNF-α, interferon-γ, interleukins as well as chemokines play an important role in inflammation [6, 12]. However, dysregulation of the inflammatory response may result in many disorders including autoimmune diseases and cancer [7].
Our results indicated that there was a significant reduction in tumor characteristics such as weight, length, depth, and volume in all treated groups in comparison to the control group. These changes may be due to the inhibition of angiogenesis and increases in tumor necrosis. Based on our recent search, no published study has investigated these outcomes so far.
Our study has some limitations. First, it would be better to evaluate the effects of more variable doses on the study parameters. Second, our study had a small sample size and future studies should be conducted on larger samples. Third, a second protein based (Western blot and immunohistochemistry) analysis was not performed to validate the RT-PCR results. Finally, regarding purified TI from Cucumis melo, our gel had too much loaded protein and it was not possible for us to perform it again due to limited financial resources. In spite of these limitations, our study has several strengths. The study duration was relatively adequate, and effects of interventions were evaluated both in vivo and in vitro. Furthermore, studies in this field have mostly studied extracts of different plant seeds. Our study was the first to evaluate both TI and extract of Cucumis melo seeds in comparison with TAM in a mouse model of breast cancer. Reverse transcription polymerase chain reaction was used to assess the gene expression of different anti-angiogenesis factors, which is a precise and validated method. We also examined a variety of doses and combinations of treatments in relation to factors related to breast cancer.
Conclusions
Trypsin inhibitor, EXT, and TAM therapy could inhibit the expression of angiogenesis-related genes such as MMP-2, MMP-9, and VEGF, and increase tumor tissue necrosis. It also caused positive changes in tumor tissue parameters such as length, width, depth, and height of the tumor with a dose-dependent effect. Combination therapy with TI and TAM had the greatest effect on reducing tumor size, inhibiting the expression of angiogenesis-related genes and tissue necrosis. Further in vivo and in vitro studies will be warranted to confirm these results and to discover the molecular mechanisms.