Introduction
Periodontal disease is a state of chronic inflammation of the tissues supporting the teeth. It is considered a civilization disease, the second most common oral health condition after tooth decay and sixth most common disease globally [1]. Untreated periodontitis may lead to oedema, gingival bleeding, alveolar bone atrophy, and consequently teeth loss [2].
Psoriasis is also considered a chronic inflammatory condition influenced by various environmental factors. It is a skin disease of unclear aetiology, typically characterized by erythematous, scaly plaques, however, it can affect multiple organ systems, such as nails and joints, which leads to the development of psoriatic arthritis [3]. Although the condition is seldom life-threatening, it poses a great physical and psychological burden, heavily impairing the quality of life. Its pathogenesis is yet not fully understood, however, the immune system is considered a key component in the development of the disease [4].
Both periodontitis and psoriasis are complex and multifactorial diseases sharing multiple risk factors, like smoking, excessive alcohol consumption, stress, and immunodeficiency [5]. They also share some of the established immunopathological mechanisms, such as a relation to the interleukin-lymphocyte IL-23/Th17/IL-17 axis [6]. One theory holds that the underlying cause of the inflammation in distant sites, such as skin and joints, is the adaptive immune response elicited by the disruption of the systemic immune balance due to oral pathogens [7]. The diseased periodontium contains inflammatory mediators, such as interleukins, prostaglandins and tumor necrosis factor-α (TNF-α), which released into the circulation, contribute to systemic inflammation [8].
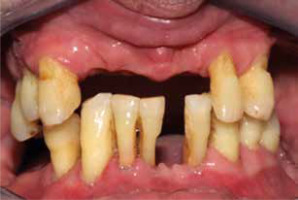
Over the last few years, extensive research has been conducted to confirm the association between periodontal disease and psoriasis. The association was established through the examination of numerous parameters, such as the number of teeth in patients, which shows an inverse correlation with the severity of psoriasis [7]. Figure 1 shows the condition of the oral cavity in one of the study participants with severe psoriasis.
It is now considered important to raise awareness of this connection among dermatologists and dentists as psoriatic lesions may indicate the presence of periodontitis and vice versa. Moreover, treatment of periodontal disease was proved to attenuate the clinical manifestations of psoriasis [8]. Diagnosis of all comorbid diseases is crucial for ensuring a comprehensive therapy [9].
Aim
The main aim of this study was to assess the correlation between the levels of selected markers of inflammation and the prevalence of psoriasis and periodontal disease. In order to collate the results with a clinical presentation, the patients were also evaluated by a dentist in terms of the condition of the gums and periodontal status, the level of oral hygiene and the frequency of dental check-ups.
Material and methods
Fifty-nine participants of both sexes aged 20-59 years were enrolled in the study. Test sample consisted of 30 patients treated in the Department of Dermatology of the Heliodor Świêcicki Clinical Hospital of the Poznan University of Medical Sciences for psoriasis. Seven of them were also diagnosed with psoriatic arthritis using the CASPAR criteria. The control group comprised 29 healthy individuals, in whom psoriasis had been ruled out by conducting thorough examination. Psoriatic patients were also subjected to examination in order to assess the severity of the disease using PASI scores, DQLI questionnaire and BSA assessments.
All the patients underwent a clinical dental examination which was performed using a dental mirror, dental probe and a periodontal probe WHO 621 Hu-Friedy scale up to 11.5 mm, under artificial lighting of the dental office. The Plaque Index (Pl.I) according to Silness and Löe [10] and the Approximal Plaque Index (API) according to Lange were used to assess oral hygiene, the Gingival Index (GI) according to Löe and Silness [11] and the Sulcus Bleeding Index (SBI) according to Muhlemann [12] were used to assess the gingival condition. Periodontal status was assessed by measuring the clinical depth of periodontal pockets (PPD) and the Clinical Attachment Loss (CAL) index.
Their blood was analysed for a selection of inflammatory cytokines (IL-1α, IL-1β, IL-6, IL-17, TNF-α) using an enzyme-linked immunosorbent assay. C-reactive protein concentrations were also measured with the MULTIGENT CRP Vario Test (CRPVa).
Results
Dental evaluation
Pl.I and API are the indices used for assessing oral hygiene. Pl.I ascertains the thickness of the plaque along the gingival margin and API is designed to express, as a percentage, the proportion of interproximal spaces covered in plaque. The differences in the medians of Pl.I and API in the test group compared to the control group were statistically significant (p = 0.007 and p = 0.028, respectively). As shown in Table 1, the median values of both indices were higher in patients with psoriasis than in the control group participants.
Table 1
Assessment of oral hygiene, gingival health and periodontal disease indices in test and control groups
As for the assessment of gingival health, indices of gingival inflammation were significantly higher in the test group than in the control one. GI classifies inflammation in three grades, based on the presence of erythema, swelling and bleeding. SBI is a scale of inflammation in which bleeding upon probing four gingival units is measured and recorded. The differences, as indicated by test results, were statistically significant (p = 0.003 and p = 0.008, respectively). The comparison of the results is shown in Table 1.
The last element of dental evaluation in clinical examination was an assessment of the overall condition of periodontium. Two indices were used, PPD and CAL, which is composed of PPD plus the distance from the gingival margin to the cementoenamel junction. Periodontal probing is one of the key elements in the diagnosis of periodontitis [13]. Both indices were much more beneficial in healthy controls than in patients with psoriasis (p < 0.001 in both measurements), which is illustrated in Table 1.
Laboratory tests
The relationship between serum levels of C-reactive protein (CRP), which were significantly elevated in psoriatic patients (Table 2), and dental indices was measured using Spearman’s correlation coefficient.
Table 2
Serum levels of CRP in test and control groups
| Variable | Test group (n = 30) | Control group (n = 29) | P-value | ||
|---|---|---|---|---|---|
| Median | Min.–max. | Median | Min.–max. | ||
| CRP [mg/l] | 6.1 | 0.2–17.9 | 0.75 | 0.14–8.4 | 0.012 |
The use of the Spearman’s coefficient demonstrated a pronounced positive correlation between CRP levels and all of the measured clinical indices (GI, SBI, PPD, CAL) (p = 0.012). However, as shown in Table 3, this correlation was present in both groups.
Table 3
Correlation between CRP levels and dental indices in test and control groups
| Variable | Test group (n = 30) | Control group (n = 29) | ||
|---|---|---|---|---|
| rs | P-value | rs | P-value | |
| CRP & GI | 0.58 | 0.001 | 0.65 | 0.0001 |
| CRP & SBI (%) | 0.72 | < 0.0001 | 0.61 | 0.0004 |
| CRP & PPD [mm] | 0.67 | 0.0001 | 0.56 | 0.001 |
| CRP & CAL [mm] | 0.64 | 0.0001 | 0.56 | 0.002 |
The evaluation of the serum levels of proinflammatory cytokines (IL-1, IL-6, IL-17A, TNF) revealed that there were no notable differences between the levels in patients with psoriasis and healthy participants (Table 4).
Table 4
Serum levels of proinflammatory cytokines in test and control groups
However, a further analysis of the available data revealed a significant correlation between a few of the cytokines (IL-1, IL-17) and dental indices (Pl.I, API, SBI) in psoriatic patients. As shown in Table 5, three out of four significant correlations transpired in the test group, which may indicate differences in the immunomodulation of the groups and confirm the association between poor oral hygiene and increased activity of the immune system.
Discussion
The relationship between psoriasis and periodontal diseases has been suspected for many years and proved by research. An epidemiological study conducted to investigate the risk of psoriasis following the diagnosis of chronic periodontitis showed that the incidence rate of psoriasis for these patients was 1.88 per 1000 person-years, compared to 1.22 in healthy controls [14]. What is more, the prevalence of periodontitis was higher in patients with more severe psoriasis [7]. However, hypotheses of the exact mechanism behind this phenomenon are mainly speculative and the impact of targeting one of the concomitant diseases on the severity of the second disease remains to be poorly understood.
The results of our study showed that the oral hygiene of patients with psoriasis was insufficient, which overall led to the worsening of their oral health. Moreover, the average number of dental check-ups in a year in this group was 0.8, while in the control group it was 1.03. The reason behind this phenomenon could be the fact that psoriatic patients might be less sensitive to periodontal pain and therefore not decide to visit a dental professional until the late stages of periodontitis [9].
Periodontal inflammation is also linked to promoting the development of a number of other systemic diseases, such as diabetes mellitus [15] or even increasing the risk of stroke [16]. It is highly important not to underestimate the role of periodontitis, one of the most common illnesses globally, in the pathogenesis of the diseases constituting the greatest concerns of public health. Periodontitis is currently believed to be a significant risk factor for cardiovascular events, such as myocardial infarction, peripheral artery disease and heart failure [17].
This study was conducted to establish whether the coexistence of psoriasis and periodontitis is associated with higher cytokine levels compared to healthy controls. The correlations observed between the levels of some interleukins in the blood and the indicators of oral hygiene, gingivitis and periodontitis give rise to hope that inflammatory markers in patients would be useful as a state-of-the-art diagnostic and even therapeutic tools. It is highly probable that novel psoriasis-targeted therapies, such as IL-17 inhibitors, would prove effective in managing chronic periodontitis [9].
Conclusions
Overall, a detailed elucidation of the relationship between psoriasis, periodontitis and markers of inflammation requires further research on larger sample sizes. However, the obtained results clearly point to the crucial role of preventing and treating periodontal diseases as a highly sufficient and cost-effective element in managing numerous systemic diseases, such as psoriasis. Patients need to fully understand that their oral health is heavily influenced by their health behaviour in the form of removing the dental plaque through regular and thorough brushing and flossing [8]. Therefore, it is important to raise awareness of the connection between periodontitis and systemic diseases among physicians and dentists as only an interdisciplinary, joint effort ensures a holistic approach to patient care.