Introduction
Pulmonary artery (PA) repair is a pivotal procedure in pediatric cardiac surgery, essential for addressing congenital heart defects that impair pulmonary circulation. Traditionally, the transannular patch (TAP) technique has been a standard approach for PA reconstruction, aimed at relieving obstruction and improving blood flow. However, this method is not without its drawbacks, particularly concerning postoperative right ventricular (RV) function and pulmonary valve (PV) insufficiency.
The use of TAP involves opening the pulmonary annulus along a commissure of the pulmonary artery and placing a patch to widen it, which can result in significant PV regurgitation and RV dysfunction. Studies have documented that TAP can lead to adverse outcomes such as RV dilatation and impaired RV function due to the loss of the valve’s dynamic role in regulating blood flow and pressure. For instance, a study by Kim et al. [1] highlighted that TAP was associated with increased risk of RV failure and reduced exercise capacity in pediatric patients, underscoring the need for alternative strategies to mitigate these risks.
In response to these challenges, innovative approaches have been explored to improve postoperative outcomes. One such technique is the use of the right atrial appendage (RAA) to construct a neovalve. This novel method has been shown to potentially mitigate some of the drawbacks associated with TAP by preserving more of the native valve’s function.
A landmark study by Amirghofran et al. [2] is among the first to report on the use of autologous RAA as a neovalve for PA repair in patients with tetralogy of Fallot. Their work, published in the European Journal of Cardio-Thoracic Surgery, demonstrated the feasibility and effectiveness of this technique. The authors provided valuable insights into the short- to mid-term outcomes of using the RAA neovalve, highlighting its potential benefits over traditional TAP methods. Their findings indicated improvements in RV function and a reduction in severe PV regurgitation, supporting the RAA neovalve as a viable alternative. Recent studies suggest that RAA neovalve construction may offer several benefits over traditional TAP.
Building on these promising results, our study aims to further investigate and compare the outcomes of PA repair using the RAA neovalve versus traditional TAP. By examining procedural metrics, RV function, and postoperative recovery, we seek to evaluate the relative benefits and limitations of these repair techniques. This research focuses on RV function, which is critical for postoperative recovery and long-term cardiac health.
Material and methods
Study design
This study is a retrospective cohort analysis conducted to compare the outcomes of PA repair using a neovalve constructed from the RAA versus TAP repair. The study focuses on evaluating key postoperative metrics, including RV function, procedural times, and recovery outcomes.
Patient selection
The study cohort included 16 pediatric patients who underwent PA repair between December 2023 and April 2024. Patients were divided into two groups based on the type of repair performed:
Surgical technique
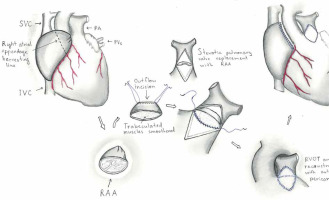
In the RAA valve group, the neovalve was constructed using a modified technique based on the method described by Amirghofran et al. [2]. After initiating cardiopulmonary bypass and achieving adequate myocardial protection, the PA was mobilized up to the bifurcation, ensuring clear exposure of the main PA. The RAA was carefully dissected while preserving its vascular supply and ensuring sufficient tissue for neovalve construction.
A longitudinal incision was made in the main PA and was extended across the annulus into the right ventricular outflow tract (RVOT) to assess the site of the obstruction or stenosis. The RAA was then tailored to create a cusp-like structure, mimicking the configuration of a native pulmonary valve. Sutures were placed along the edges of the RAA tissue and the incision in the PA to secure the neovalve in position, ensuring that the leaflet tissue provided adequate coaptation during systole and diastole. Particular care was taken to avoid tension or distortion of the RAA tissue.
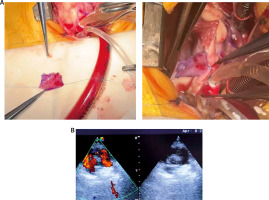
The neovalve was tested intraoperatively by flushing saline through the PA to verify its competence and functionality. After ensuring satisfactory valve performance, the PA was reconstructed over the neovalve using autologous or synthetic patches as necessary. Hemostasis was meticulously achieved before weaning the patient off cardiopulmonary bypass.
For the TAP group, the standard transannular patch technique was employed to relieve the obstruction in the PA. Following exposure of the stenotic area, a longitudinal incision was extended across the annulus into the right ventricular outflow tract (RVOT). A patch, tailored from autologous pericardium or synthetic material, was sutured in place to widen the RVOT and PA. Postoperative echocardiography confirmed the patency of the reconstructed RVOT and PA.
Figures 1 and 2 illustrate the steps of the neovalve construction process and its intraoperative appearance.
Data collection
Data were collected from medical records, including:
demographic information: age, sex, weight, height,
preoperative details: history of Blalock-Taussig (BT) shunt, PA annulus size, and Z-score,
procedural metrics: procedure time, bypass time, and aortic cross-clamp (ACC) time.
Postoperative outcomes: mortality, intensive care unit (ICU) stay, hospital stay, PV regurgitation, RV dysfunction, and RVOT peak gradient.
To ensure comparable groups and minimize selection and performance bias, we included 3 patients in the TAP group who had undergone a BT shunt via left thoracotomy approximately 1 year prior, matching the 3 patients in the RAA neovalve group who had also undergone the same procedure.
Statistical analysis
All statistical analyses were performed using the Jamovi application. Continuous variables were compared using the independent t-test, while categorical variables were analyzed using the c2 test. Pearson’s correlation coefficient was used to assess the relationship between PV regurgitation and RV dysfunction. A p-value of < 0.05 was considered statistically significant.
Ethical considerations
This study was conducted as a retrospective cohort analysis. Given the nature of the study, which involved the review of existing medical records without direct patient intervention or interaction, informed consent was not required. The study was approved by the Institutional Ethics Committee of Tashkent Pediatric Medical Institute (#2, 25.03.2024y), which determined that the use of de-identified patient data for research purposes did not necessitate patient consent.
Results
The study included 16 pediatric patients undergoing PA repair, divided into two groups: those receiving the RAA valve (n = 8) and those undergoing traditional TAP (n = 8). Both groups were comparable in terms of age, sex distribution, weight, height, history of BT shunt, PA annulus size, and Z-score (Table I).
Table I
Patient demographic and clinical variables for the right atrial appendage (RAA) neovalve and transannular patch (TAP) groups
The average procedure time was longer in the RAA valve group (222 ±33.1 minutes) compared to the TAP group (196 ±16.7 minutes), but this difference was not statistically significant (p = 0.07). In contrast, the TAP group had a significantly shorter bypass time (126 ±6.48 minutes vs. 145 ±10.0 minutes, p < 0.001) and ACC time (86.6 ±3.7 minutes vs. 104 ±10.3 minutes, p < 0.001) compared to the RAA valve group (Table II).
Table II
Procedural metrics and postoperative outcomes for right atrial appendage (RAA) neovalve and transannular patch (TAP) groups
Postoperative outcomes showed that patients in the RAA valve group had significantly shorter ICU stays (3.88 ±0.84 days) and hospital stays (10.1 ±1.25 days) compared to those in the TAP group (ICU stay: 6.13 ±2.75 days, p = 0.044; hospital stay: 13.2 ±3.19 days, p = 0.029). The mortality rate was higher in the TAP group (25%), but this difference was not statistically significant (p = 0.131) (Figure 3).
Figure 3
Violin plots showing the distribution of intensive care unit and hospital stay durations for the right atrial appendage (RAA) neovalve (group 1) and transannular patch (TAP) groups (group 2)

Regarding postoperative complications, the RAA valve group had significantly fewer instances of severe PV regurgitation compared to the TAP group. Specifically, all patients in the RAA valve group had no or mild regurgitation, whereas the TAP group had several patients with moderate to severe regurgitation (p < 0.001).
RV function was also notably better in the RAA valve group. More patients in this group had no RV dysfunction (4 patients) compared to the TAP group (none, p = 0.014). Additionally, the RAA valve group had a higher RVOT peak gradient (25.3 ±6.1 mm Hg) compared to the TAP group (16.9 ±7.9 mm Hg, p = 0.032), indicating a significant difference in RV outflow (Table II).
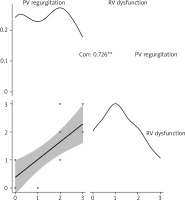
A significant positive correlation was found between PV regurgitation and RV dysfunction, with Pearson’s correlation coefficient (r) calculated at 0.726, indicating a strong relationship between these two variables (p < 0.001). This suggests that higher levels of PV regurgitation are associated with greater RV dysfunction in our cohort (Figure 4).
Discussion
This study compares outcomes between PA repair using a neovalve constructed from the RAA and TAP repair. Our findings suggest that the RAA neovalve technique may offer several advantages over TAP, particularly regarding postoperative recovery and RV function. The RAA neovalve group showed significantly fewer cases of severe PV regurgitation and better RV function compared to the TAP group.
Traditional TAP repair has been a common approach for PA reconstruction, as it allows for adequate outflow tract size but is associated with significant postoperative complications. Previous studies [1, 3, 4] have documented that TAP is linked with a higher incidence of RV dysfunction and PV regurgitation. The chronic nature of these issues often necessitates further interventions and affects long-term outcomes. Alipour Symakani et al. [5] investigated RV function at the tissue level in patients after surgical correction of tetralogy of Fallot (ToF) and concluded that ‘as long as the long-term outcome of surgical correction of tetralogy of Fallot remains suboptimal, other treatment strategies need to be explored’. This underscores the importance of examining innovative methods, such as the RAA neovalve, to reduce RV dysfunction and address RVOT obstruction.
In contrast, the use of autologous tissues for valve reconstruction, as explored in recent studies, has shown promise in mitigating some of these complications. For instance, Amirghofran et al. [2] conducted one of the first comprehensive studies on using RAA for neovalve construction, demonstrating its potential to reduce PV regurgitation and improve RV function. Their findings indicate that the RAA neovalve can provide a more physiological valve replacement, which aligns with our results showing fewer severe PV regurgitation cases and improved RV function.
Other recent studies have also explored alternative techniques to address the limitations of TAP. For example, a study by Cocomello et al. [6] highlighted the benefits of using homograft valves and their impact on RV function and patient outcomes. Similarly, work by Abbas et al. [7] on various surgical techniques for RV outflow tract reconstruction underscored the importance of optimizing valve function to improve postoperative recovery.
In our study, while the RAA valve group had a longer procedure time compared to the TAP group, this difference was not statistically significant. However, the TAP group had significantly shorter bypass and aortic cross-clamp (ACC) times, which may reflect the procedural differences and complexity associated with RAA valve construction. Despite the longer procedural time, the RAA valve group demonstrated significantly shorter ICU and hospital stays. This advantage may be attributed to the lower incidence of severe PV regurgitation and better RV function observed in this group, which aligns with findings from studies such as those by Schulte et al. [8], who reported improved recovery outcomes with advanced valve repair techniques.
The significant correlation between PV regurgitation and RV dysfunction (r = 0.726, p < 0.001) observed in our study further emphasizes the critical impact of PV regurgitation on RV function. This finding aligns with previous research that highlighted the detrimental effects of severe PV regurgitation on RV performance. For instance, other authors [2, 8] also reported similar associations, suggesting that strategies to reduce PV regurgitation, such as the use of the RAA neovalve, could be pivotal in preserving RV function and improving postoperative outcomes. The strong correlation we found underscores the importance of minimizing PV regurgitation to prevent RV dysfunction, which is crucial for long-term patient prognosis.
Implications and future directions
The promising outcomes associated with the RAA neovalve technique suggest that using autologous tissue for valve reconstruction may offer a more physiological solution than traditional TAP. The improved RV function and reduced PV regurgitation observed in our study are consistent with the benefits reported in the literature. For example, research by Cocomello et al. [6] on autologous and bioprosthetic valves highlighted the potential for these approaches to reduce complications and improve functional outcomes.
Despite these encouraging results, our study is limited by its small sample size and retrospective design. Larger, prospective studies are needed to validate our findings and further assess the long-term benefits of the RAA neovalve technique. Future research should also focus on the impact of this technique on functional outcomes, quality of life, and the need for additional interventions.
Conclusions
The RAA neovalve technique for PA repair shows significant promise as an alternative to traditional TAP, with favorable outcomes related to RV function and postoperative recovery. The reduced incidence of severe PV regurgitation and improved recovery times underscore the potential advantages of using autologous tissue for valve reconstruction. As surgical techniques continue to evolve, the RAA neovalve may become a valuable option for improving outcomes in pediatric cardiac surgery.