Dear Editor,
Recent years have seen increasing interest in the use of extracorporeal life support (ECLS) in the context of acute hypercapnic respiratory failure due to near-fatal asthma and chronic obstructive pulmonary disease [1, 2]. We describe a case of ECLS use as a rescue measure in a patient with infective exacerbation of chronic bronchiectasis (Kartagener’s syndrome).
A 43-year-old man was admitted with complaints of fever associated with chills and rigor for 10 days, cough with purulent expectoration for one week, with worsening shortness of breath for 2 days preceding admission. His medical history was significant for bronchiectasis, sinusitis, and complete situs inversus as part of Kartagener’s syndrome. He had recovered from COVID-19-related pneumonia one year before the current admission.
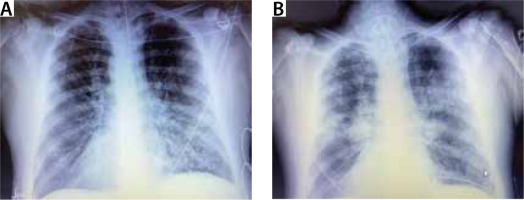
He was conscious and oriented at the time of admission but severely tachypneic, using accessory muscles of respiration, and he had a silent chest on auscultation. Hypercarbia and hypoxia were worsening despite continuous nebulization with bronchodilators, O2 support, steroids, and other supportive therapy. After a brief trial of non-invasive ventilation (NIV), the patient was intubated on arrival to the intensive care unit due to further CO2 cumulation, progressive drow-siness, and impending respiratory arrest. Severe bronchospasm, refractory to nebulization, steroids, magnesium, and aminophylline infusion persisted. Copious amounts of purulent secretions were present, requiring frequent suctioning. Chest radiograph showed patchy non-homogeneous opacities (Figure 1). Post intubation his airway pressures remained extremely high (ACMV mode, TV 400 mL, PEEP 5, RR 18 min–1, FiO2 0.6, Ppeak around 60–70 cm H2O, compliance 24– 28 mL cm–1 H2O–1, resistance 22– 24 cm H2O L–1 s–1). He started developing haemodynamic instability requiring dual vasopressor support (norepinephrine 0.35 μg kg–1 min–1 and vasopressin 2.4 IU h–1) despite optimizing ventilatory settings for severe expiratory flow limitation. He was started on an intravenous keta-mine infusion, to no avail, after which he was given inhaled sevoflurane therapy because of persistent refractory spasms. Despite all the supportive therapy for relieving spasm, reducing secretions, and causing bronchodilation, the patient’s condition continued to deteriorate, and his haemodynamic condition remained unstable (probably secondary to the severe air trapping). His blood gases showed severe respiratory acidosis with hypoxaemia, while his laboratory results showed an elevated white cell count (Tables 1 and 2). After discussion with his family, it was decided to initiate VV-ECMO therapy as a rescue measure to reduce the adverse effect of air trapping on blood pressure, reduce the ventilatory requirements, and allow for the infection and bronchospasm to resolve (Figure 2).
FIGURE 2
Flow chart depicting the course from admission to the decision to implement extracorporeal life support (ECLS)
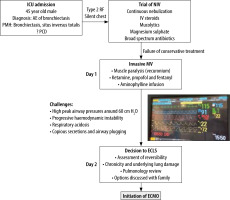
TABLE 1
Arterial blood gas values during ICU stay
TABLE 2
Laboratory values during ICU stay
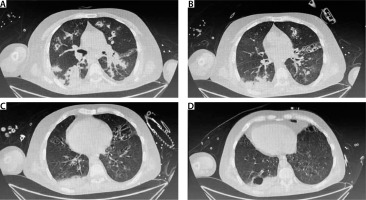
The pros and cons of VV-ECMO therapy including the need for anticoagulation, adverse effects including bleeding, risk of prolonged thera-py, and lack of resolution due to the pre-existing structural lung damage and congenital condition (ciliary dysmotility) were explained to the family in detail. He underwent VV ECMO cannulation (Maquet Rotaflow, right femoral-right jugular configuration, 25 Fr drainage, and 20 Fr return can-nula). The initial ECLS settings were as follows: 3000 rpm, pump blood flow of 4 litres per minute (Lpm), and sweep gas of 8 Lpm. Anticoagulation with unfractionated heparin was titrated to ACT values. Following the commencement of ECLS, his respiratory acidosis was gradually corrected over the next 24 hours. Arterial blood gases were performed post commencement of ECLS, 30 minutes after any change in sweep gas flow, and every 6 hours for the first 24 hours, targeting a PaCO2 reduction of less than 50% in the first 24 hours of ECLS initiation from the pre-ECLS pCO2 of 111 mmHg (Table 1). Subsequent sweep gas titrations were based on the ability to achieve better CO2 removal following resolution of pneumonia and bronchospasm. ECLS allowed ultraprotective mechanical ventilation (ACMV mode, FiO2 0.4, tidal volume 300 mL, PEEP 8, RR 12 breaths min–1, I : E ratio 1 : 3), and he was weaned off the vasopressor medications over the next 24 hours. His endotracheal aspirate cultures grew MDR Klebsiella pneumonia, and antibiotic therapy was modified accordingly. He underwent percutaneous tracheostomy due to significant neuromuscular weakness and anticipated prolonged ventilation and difficulty in clearing secretions. He was decannulated on day 9 of ECLS support. He was gradually weaned off the ventilator by day 15 of the ICU stay and transferred to the internal medicine ward. He was discharged home on day 24. A follow-up HRCT chest was performed, which showed bilateral bronchiectatic changes along with multifocal patchy areas of confluent alveolar densities/centrilobular nodules in bilateral lung fields (Figure 3).
DISCUSSION
Patients with severe bronchiectasis in the intensive care unit tend to have poorer outcomes with advanced age, lower premorbid activity index, and inotropic support being important determinants of mortality [3, 4]. The management of the above case presented us with unique challenges (Table 3). The initial management of acute exacerbation of his pre-existing bronchiectasis was on conventional lines [5, 6]. He had severe wheezing and copious purulent sputum production on admission, which necessitated the use of aggressive bronchodilator therapies, mucolytics, anti-inflammatory drugs, and broad-spectrum antibiotics. A trial of NIV may prove beneficial in such patients and was tried but eventually escalated to invasive ventilation following signs of impending respiratory arrest [7].
TABLE 3
Challenges in patient management
Invasive ventilation, however, proved to be extremely challenging due to severe expiratory flow limitation, dynamic hyperinflation, and air trapping. Delivery of 4–6 mL kg–1 tidal volume needed peak pressures ranging from 60 to 70 cm H2O. In addition to deep sedation, ketamine infusion and inhaled sevoflurane were tried given the beneficial effects on bronchodilatation, improving expiratory flow rate and gas exchange [8, 9]. However, copious purulent sputum production seemed to be a significant deterrent as episodes of severe spasm with fluctuation in oxygenation levels relapsed. Secondarily, his haemodynamic parameters continued to worsen secondary to severe air trapping, respiratory acidosis, and high ventilating pressures and consequent right ventricular pressure overload.
The decision to offer ECLS as a rescue measure was based on a review of his recent chest scans and agreement between the treating intensivist and pulmonologist regarding the possible reversibility of his underlying disease process once his pneumonia was adequately treated. ECCO2R (extracorporeal carbon dioxide removal) may be an ideal technique in such cases due to the smaller priming volume and lower extracorporeal blood flow [10]. We chose ECMO due to the non-avail-ability of ECCO2R in our setup. The chal-lenges of ECLS cannulation in a patient with possibly altered vascular anatomy due to situs inversus cannot be overemphasized. However, in this case, the right internal jugular vein central catheter seemed to follow a straight course to the heart, which allowed us to use the same vein for placement of the return cannula. Rajab et al. [11] similarly described the use of the right internal jugular vein for ECLS cannulation in a patient with dextrocardia.
Timely initiation of ECLS obviated the harmful effects of invasive mechanical ventilation with lower peak pressures and respiratory rate, decreased air trapping, and improved gas exchange parameters, and thereby reduced the adverse effect of high ventilatory pressures and air trapping and hypercarbia and respiratory acidosis on the right heart. The patient’s haemodynamic parameters continued to improve, allowing for withdrawal of vasopressor support within 24 hours. ECLS has been used successfully in patients with obstructive airway disease to avoid invasive ventilation and to facilitate weaning, and it has shown beneficial effects on haemodynamic parameters due to resolution of air trapping and acidosis [12–14]. The PaCO2 was gradually decreased in the initial 24 hours of ECLS with frequent blood gas monitoring and titration of sweep gas flow given the hazards of overly rapid correction [15]. We encountered a relatively long ECLS run of 9 days, due to the longer time needed for resolution of pneumonia and bronchospasm, similarly to a report by Brodie et al. [16].
The management of MDR pneumonia, however, continued to be challenging, requiring clearance of secretions by repeated bronchoscopy, intense chest physiotherapy and broad-spectrum inhaled, and intravenous antibiotic therapy. He was assessed every 24 hours for the feasibility of weaning from ECMO (day 5 of ECLS support onwards) with a drop in sweep gas and adjustment of ventilatory support once we felt that his pneumonia was showing signs of resolution.
Our experience highlights the potential role of ECLS in a complex patient with an infective exacerbation of chronic bronchiectasis with severe flow limitation. While ECLS would not be the first-line therapy, it could prove to be lifesaving if instituted judiciously and following the failure of conventional mechanical ventilation and supportive measures to achieve physiological homeostasis.