Strokes in mechanistic relation to carotid atherosclerosis are often large and disabling [1]. Evidence shows that pharmacologic therapy (even if maximised) is not sufficient to universally prevent carotid-related strokes [2]. Revascularisation continues to play an important role in particular in increased-stroke-risk carotid lesions [2, 3], with carotid artery stenting (CAS) as a minimally invasive technique in primary and secondary prevention of carotid-related stroke [2, 3].
Carotid atherothrombotic plaques are often fragile; thus, any plaque manipulation generates embolic material. Both CAS and carotid surgery are (and will remain) embologenic [4]. Unprotected CAS carries the risk of cerebral embolism at each of the key stages of the procedure, from the lesion crossing with a wire, through stenosis predilatation, stent positioning and implantation, and stent post-dilatation [5]. Several cerebral embolic protection strategies have been developed to improve the safety of CAS, including distal filter devices and transient flow arrest/reversal devices [5].
Filter use is associated with “unprotected” crossing of the lesion and other limitations such as risk of suboptimal filter apposition to the arterial wall, embolism with particles smaller than the filter pores (~100–180 µm) and limited filter basket capacity [5]. However, filer use is intuitive, it allows maintained visualisation throughout the procedure, and it remains to be the preferred mode of embolic protection by majority of CAS operators [6]. In the case presented (Figure 1), when discussing the procedure strategy in the context of clinical presentation, baseline ultrasound imaging examination and baseline angiogram (LINC – Leipzig Interventional Course 2024; Session “20 years of innovation: Best practice in carotid revascularization”; P. Musialek: Carotid revascularisation in 2024: Key factors to consider), the majority of operators declared a preference for using a filter protection. The results of the poll were the following: CAS with filter protection – 76.2%, CAS with proximal system – 6.3%, surgery – 5.3%, pharmacological treatment only – 1.1%, no clear preference – 11.1%.
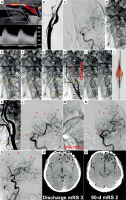
Figure 1
Filter-protected CAS demonstrating limitations of filter protection despite anti-embolic stent use. A 59-year-old man was admitted for carotid revascularisation 2 weeks after left-hemispheric TIA.
Duplex Doppler ultrasound examination showed a fibro-lipidic atherosclerotic plaque in LICA causing a significant stenosis (A). LCCA/LICA angiogram showed a significant LICA stenosis with a visible contrast channel (B). Baseline left cerebral angiogram is shown in C. In absence of Mo.Ma proximal protection on-shelf availability a decision was made to use a distal (filter) cerebral protection and an (routine) anti-embolic stent. The filter was uneventfully delivered through the lesion and was opened in distal LICA (D – white arrowhead). A gentle predilatation was performed with a small coronary balloon (E). There was an acute deterioration of the patient neurologic status with impairment of responsiveness. An anti-embolic stent was implanted (F) and routinely optimised (G). However, “no flow” was visible in the treated artery upon contrast injection (H), consistent with filter blockage by embolic material. Acute neurologic symptoms of left cerebral hemispheric ischaemia were aggravating. To minimise the risk of distal embolism, the filter was removed in a “half-open” position (I). The filter examination showed macroscopic evidence of embolic material (J). Carotid completion angiogram (K) demonstrated an optimal angiographic result at the level of carotid bifurcation. Cerebral angiogram (L) demonstrated multiple distal cerebral artery embolic lesions as well as embolism of the anterior communicating artery (red arrowheads). The latter, however, had a good compensatory filling from the contralateral (right) side; thus, no anterior communicating artery thrombectomy was considered indicated. RtPA (10 mg) was administered to distal LICA via a microcatheter (M), but no symptom improvement (and no cerebral angiogram improvement) occurred over 45 min, consistent with athero-embolic rather than thrombo-embolic mechanism of the multiple distal vessel occlusion. The patient developed a multi-site left hemispheric infarct (N, M – black arrowheads) with clinical symptoms of an acute procedure-related stroke. Discharge mRS (day 9) was 3. On clinical examination at 90-days the patient had residual neurologic deficit but was functionally independent (mRS 2). There were permanent multi-site chronic infarcts on cerebral plain CT (R) that corresponded to the (sub)acute cerebral lesions depicted in P.
Note that filter use in endovascular carotid revascularisation may be associated with distal embolism risk due to (1) unprotected crossing of the lesion, (2) potential filter basket malapposition, (3) embolism by particles < filter pore size, and (4) limited filter basket capacity [5]. On the other han, the anti-embolic stent cerebral protection is exerted only after the stent full deployment and optimisation – but it then extends throughout the stent healing period. These filter limitations do not apply to proximal protection by transient flow cessation or reversal [5, 7, 9, 15]
CT – computed tomography, mRS – modified Rankin scale, LCCA – left common carotid artery, LICA – left internal carotid artery, TIA – transient ischaemic attack.
Proximal protection, in comparison with filter protection, reduces the magnitude of cerebral embolism by ≈10- to 30-fold at the stages of lesion wiring, predilatation, stent positioning and deployment, and stent postdilatation [7]. Proximal embolic protection is associated not only with a lower incidence and magnitude of procedural cerebral microembolisation but also with a lower incidence of cerebral adverse events [8]. Proximal systems, if appropriately used, allow zero intraprocedural cerebral embolism [9], but they are less intuitive and require training for a competent application [5, 9, 10]. Recent analysis from the ACST-2 trial showed filter use in 80% protected CAS procedures (proximal devices – 20%, including mostly the Mo.Ma system) [6]. Proximal embolic protection requires operator familiarity with the system and proficiency in its application, including a learning curve. This is compensated with the proximal protection capacity to capture debris of all sizes from the point of lesion crossing to the point of stent postdilatation optimisation.
We demonstrate a very rare but serious cerebral embolic complication that occurred with filter protection use in absence of on-shelf availability of the Mo.Ma system [5, 7]. In the presented case, multi-focal cerebral iatrogenic embolism was not amenable to mechanical intervention (distal-vessel occlusions) – as primarily non-thrombotic – and it did not respond to administration of 10 mg rtPA via the distal internal carotid artery (Figure 1).
In the case presented, the MicroNET-covered anti-embolic stent was selected to extend neuroprotection throughout the period of stent healing. This second-generation carotid stent has sealing properties [11–14] and level-1evidence for a significant reduction of intra-procedural plaque-related cerebral embolism and elimination of post-procedural ischaemic events. It is important to note that the anti-embolic stent will exert its cerebral embolism prevention only from the point of its implantation and full optimisation [5]. Proximal/distal protective devices and anti-embolic stents play complementary roles in cerebral protection and cannot be replaced by each other, and they are not to be used interchangeably [5, 10, 15].
In conclusion, the role of embolic protection devices is maintained in the era of anti-embolic stent use. There remains a need to take advantage of the unique features of proximal embolic protection systems (including their potential to completely prevent cerebral ischaemic events) [5, 7, 9, 10], particularly when treating lesions of increased embolic risk. CAS operators should be cognisant of the fact that the anti-embolic stent cannot be expected to exert any effect until implanted and post-dilated [15]. Practical knowledge and experience on how to use proximal cerebral protection to reduce intra-procedural embolic complications of CAS is an indispensable element of today’s competent CAS [5, 7].








