Introduction
Left main (LM) trifurcations are encountered in about 10–20% of LM cases and may require specific treatment strategies [1].
Aim
The aim of this retrospective analysis was to assess the effectiveness and safety of percutaneous coronary interventions with deployment of drug-eluting stents (DES) (both regular drug-eluting stents (rDES) and dedicated bifurcation BiOSS stents) in LM trifurcations in patients with stable coronary artery disease (CAD) and non-ST elevation acute coronary syndrome (NSTE-ACS).
Material and methods
Study population
We retrospectively analyzed data from the BiOSS Expert Registry, the international randomized clinical trial POLBOS I with BiOSS Expert, the BiOSS LIM Registry and the international randomized clinical trial POLBOS I with BiOSS LIM [2–6]. Patients with a final diagnosis of stable CAD or NSTE-ACS were enrolled between 2010 and 2013 in centers in Poland, Spain and Bulgaria. All patients signed the informed consent. Patients with STEMI or Medina type 001 bifurcation lesions were excluded from the registry. Provisional T-stenting (PTS) was the obligatory strategy.
The BiOSS is a coronary, dedicated balloon-expandable bifurcation stent. The platform is made of 316L stainless steel (strut thickness 120 µm) and the stent is coated with a biodegradable polymer that elutes paclitaxel (BiOSS Expert – paclitaxel concentration 1 µg/mm2) or sirolimus (BIOSS LIM – sirolimus concentration – 1.4 µg/mm2). The BiOSS stent consists of two parts, proximal and distal, joined with two connection struts at the middle zone [7]. In the rDES group, the use of any approved rDES available in participating catheterization laboratories was allowed. The POLBOS I trial started in 2010 when paclitaxel-eluting stents were routinely used. The following regular paclitaxel-eluting stents were used: LucChopin2 (1 µg/mm2, Balton), Coroflex Please (1 µg/mm2, B. Braun), Taxcor (1 µg/mm2, Eurocor) and Apollo (1 µg/mm2, IK), whereas olimus-eluting stents were as follows: everolimus-eluting stents (Xience, Abbott Vasc; Promus, Boston Scientific), sirolimus-eluting stents (Cypher, Cordis; Prolim, Balton; Orsiro, Biotronik; Cre8, CiD), biolimus-eluting stents (Biomime, Biomime; Biomatrix, Biosensors) and zotarolimus-eluting stents (Resolute Integrity, Medtronic).
Follow-up
Clinical follow-up was performed with office visits or by telephone at 12, 24, 36, 48 and 60 months after the intervention. Adverse events were monitored throughout the study period.
Endpoints
The primary endpoint was the cumulative rate of major adverse cardiovascular events (MACE) consisting of cardiac death, myocardial infarction (MI) and target lesion revascularization (TLR). Secondary endpoints included cardiac death, all-cause death, MI, TLR, target vessel revascularization (TVR), stent thrombosis (ST), and device success [8]. Cardiac death included death resulting from an acute MI, sudden cardiac death, death due to heart failure, and death due to cardiac procedures. All deaths were deemed cardiac unless proven otherwise. Myocardial infarction was defined according to the third universal definition [9].
Statistical analysis
Continuous variables were presented as mean ± SD. Categorical data were presented as numbers (%). Continuous variables were compared using an unpaired Student two-sided t test, and categorical data using the χ2 test or Fisher’s exact test, as appropriate. If the distribution was not normal (verified with the Shapiro-Wilk test), the Wilcoxon signed-rank test and the Mann-Whitney U-test were used. The analysis of time to event was performed with the Kaplan-Meier estimator of survival curve. A log-rank test was used to compare survival distributions. P-values of < 0.05 were considered statistically significant. Statistical analyses were performed using R 3.0.2 for OS (R Foundation, Vienna, Austria).
Results
The analyzed population consisted of 245 patients, in whom 189 patients had a BiOSS stent deployed in the distal LM and 56 patients rDES. In this population we identified 178 (72.7%) cases with distal LM bifurcation and 67 (27.3%) cases with distal LM trifurcation. Additionally, we differentiated three types among trifurcations: true trifurcations treated with only one stent (BiOSS or rDES) (type I: n = 12, 17.9%), true trifurcations treated with 2- or 3-stent technique (type II: n = 36, 53.7%) and pseudo-trifurcations with high take-off of diagonal or marginal branches (type III: n = 19, 28.4%). The mean age of enrolled patients (82.1% males) was 66.8 ±9.77 years. All stents were implanted successfully (mean pressure 12 atm). In 45 (67.2%) cases the second stent was implanted within the side branch, mainly using T and protrusion (TAP) technique (including 9 cases from type III). In 64.2% of cases procedures were performed from radial access, and 20.9% using a 7 Fr guiding catheter. Detailed characteristics are presented in Table I.
Table I
Baseline and procedural characteristics
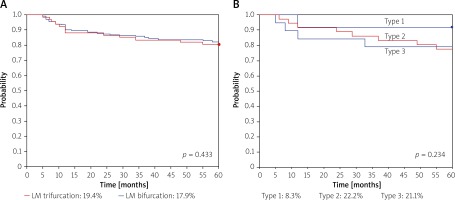
In 14 (20.9%) cases periprocedural MI was diagnosed. At 12 months there were no statistically significant differences between LM trifurcation types in MACE rates, 0, 11.1% (n = 4), 10.5% (n = 2), respectively. At 5-year follow-up the MACE rate for the whole study group was 19.4% (n = 13), whereas MACE rates for type 1, type 2 and type 3 were 8.3%, 22.2% and 21.1%, respectively (Figure 1, Table II). No significant differences were observed between BiOSS and rDES subgroups (data not shown). Among 10 cases of TLR CABG was done in 2 cases, PCI with DES in 5, and PCI with drug-eluting balloon (DEB) in 3 cases.
Discussion
There are scarce data in the literature on the treatment of trifurcation left main disease. This is currently one of the largest reported cohorts of patients with unprotected LM stenosis treated with PCI and DES implantation with the longest follow-up [10]. Most studies have 3-year follow-up or assess bare metal stents; therefore it is difficult to compare MACE rates [10–12]. Nevertheless, our results of LM trifurcation stenting are in agreement with previous studies [13, 14]. The overall relatively high rate of adverse events was mostly driven by a high TLR rate. There was no significant difference compared to distal unprotected LM bifurcation treatment (Figure 1, Table II).
Limitations
The use of multiple stent types and drugs among rDES was a limitation, although this aspect of the design was intended to replicate real-world clinical practice. Also, the low number or rDES might influence the final outcome data compared with dedicated bifurcation stents.