Summary
Despite significant technical progress, patients treated within chronic total occlusions (CTO) using percutaneous coronary intervention (PCI) are at increased risk of periprocedural complications. The main finding of the current study is that patients from the CTO PCI group presented a significantly higher frequency of periprocedural complications and coronary artery perforations (CAPs) in comparison to the non-CTO PCI group. The frequency of overall periprocedural complications and CAPs remained stable in the CTO PCI group during the assessed period of time, while in the non-CTO PCI and all-comers groups, the overall periprocedural complication rate significantly decreased and the CAP rate significantly increased in the all-comers group.
Introduction
Despite significant technical progress, patients treated within chronic total occlusions (CTO) using percutaneous coronary intervention (PCI) are at increased risk of periprocedural complications [1, 2]. CTO PCI is a strong predictor of coronary artery perforation (CAP) in the all-comers group of patients treated with PCI [3]. The incidence of CAP during PCI is estimated at 0.1–0.84% [4, 5]. CAP can have life-threatening consequences which include tamponade, major bleeding, the need for emergent cardiac surgery and in-hospital death [6, 7]. The use of PCI in higher risk subgroups, especially among older patients, has been recently increasing [8]. Parsh et al. reported that among 181,590 patients treated with PCI, 625 (0.34%) suffered CAP during the PCI procedure and 41 (6.56%) patients with perforation died before discharge. While the frequency of CTO PCI cases has been reported to increase significantly over the last years, from 1.6% to 2.8% of all cases, the incidence of perforation did not show a statistically significant trend [3]. Although the incidence and predictors of CAP have recently been studied in several large all-comers PCI series, limited data exist on predictors of CAP during CTO PCI [3, 9, 10]. CAPs in PCI occur rarely, but are more frequent during CTO PCI (1.4% to 4.4%) and among retrograde PCIs [6, 7, 10]. Increased mortality at follow-up after CAP has been recently reported [5]. CTO PCI success rates continue to improve as new techniques and tools develop. The occurrence of periprocedural complications, however, continues to affect risk-benefit considerations, and is estimated at 3.1% in a large meta-analysis [11]. So far, it has been shown that PCI within CTO in post-interventional follow-up mainly improves the reduction of clinical symptoms of ischaemia, while it has a lesser impact on survival; however, a “practice-based medicine” approach often dominates in everyday practice rather than “evidence-based medicine” [1, 6, 11].
Aim
To investigate the frequency of periprocedural complications, with special insight into CAPs among patients treated with PCI within CTO and their predictors.
Material and methods
Study design and patient population
This retrospective analysis was performed on prospectively collected data. Data for conducting the current study were obtained from the National Registry of Percutaneous Coronary Interventions (ORPKI). The registry has been described in previously published papers [12]. Data were collected from the registry between January 2014 and December 2018. We selected 12,572 patients treated with PCI within CTO out of 535,853 patients treated using PCI during the analysed period. The technical aspects of the procedure such as the choice of access site (femoral or radial), culprit lesion approach (antegrade or retrograde), sheath and catheter size, as well as guidewires, microcatheters and other devices specific for CTO PCIs, were at the operator’s discretion. A coronary CTO is defined as a total occlusion in a coronary artery with non-collateral Thrombolysis in Myocardial Infarction (TIMI) flow grade 0 of at least 3-month duration [13]. Furthermore, the periprocedural anticoagulation and indications for PCI as well as stent type also remained at the first operator’s discretion. Antiplatelet therapy was implemented according to current European Guidelines [14]. The protocol complied with the 1964 Declaration of Helsinki, and all participants provided their written informed consent for the percutaneous intervention. Due to the retrospective nature and anonymisation of the collected data and registry, obtaining the consent of the Bioethics Committee was not required.
Endpoints of interest
Primary endpoints of interest were periprocedural complications, which consisted of arterial dissections, CAPs, cardiac arrests, deaths, allergic reactions, myocardial infarctions, no-reflows, cerebral strokes and puncture-site bleedings. The identification of individual complications was left to the discretion of the operator and included those which occurred within the catheterization laboratory. The no reflow phenomenon was defined when angiographic evidence of reopening of the occluded coronary artery and successful stent placement with no evidence of flow-limiting residual stenosis (< 50%), dissection, vessel spasm, or thrombus burden was present and concomitant Thrombolysis in Myocardial Infarction (TIMI) flow grade ≤ II, or a TIMI flow grade III with a myocardial perfusion grade 0 or I, at least 10 min after the end of the PCI procedure was found. Prior to propensity score matching (PSM), we calculated predictors of overall periprocedural complications and CAPs in the all-comers group of patients treated with PCI. After PSM, we also assessed the effect of PCI within CTOs on the overall periprocedural complication count as well as other particular complications.
Statistical analysis
Categorical variables are presented as numbers and percentages. Continuous variables are expressed as mean (standard deviation (SD)) or median (interquartile range (IQR)), where applicable. Normality was assessed via the Shapiro-Wilk test. Equality of variance was evaluated using Levene’s test. Differences between the two groups were compared using Student’s or Welch’s t-test depending on the equality of variances for normally distributed variables. The Mann-Whitney U test was applied for non-normally distributed continuous variables. Categorical variables were compared with Pearson’s χ2 or Fisher’s exact test if 20% of cells had an expected count of less than 5 (Monte Carlo simulation for Fisher’s test using tables of higher dimensions than 2x2). Multifactorial logistic regression models were constructed to find predictors of all procedure-related complications and CAPs in the all-comers group of patients treated with PCI. Then, all of the baseline/demographic characteristics were included in the logistic regression model via PSM. PSM was performed with the nearest neighbour algorithm. The groups were considered balanced if standardised differences for each of the analysed baseline/demographic characteristics were lower than 10%. The PSM included age, body mass, diabetes, prior stroke, prior myocardial infarction (MI), prior PCI, prior coronary artery bypass grafting (CABG), smoking status, arterial hypertension, kidney disease, chronic obstructive pulmonary disease (COPD), vascular access, thrombectomy, rotablation use, TIMI flow grade before PCI, gender, clinical presentation at baseline, cardiac arrest before admission to hospital, the use of imaging modalities (fractional flow reserve (FFR), intravascular ultrasound (IVUS), optical coherence tomography (OCT)), results of coronary artery angiography, type of PCI and culprit lesion. FFR was used to assess other borderline stenosis in target vessel (CTO treated with PCI) located before or behind the culprit lesion and measured after PCI. The effect of PCI within CTO on procedure-related complications was assessed using mixed-effect models to account for matching. Statistical analysis was performed using R, version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria, 2019) with the following packages: ‘MatchIt’, version 3.0.2 and ‘lme4’, version 1.1-21.
Results
Population and complications
Before PSM, we compared the group of 523,281 patients treated with PCI to the non-CTO culprit lesion with 12,572 patients treated within the CTO culprit lesion. All complication frequencies were significantly higher among patients in the CTO PCI group compared to the non-CTO PCI group (p < 0.001, Table I). This was mainly caused by the significantly higher frequencies of no-reflow (p = 0.02), CAP (p < 0.001) and puncture-site bleeding (p < 0.001) in the CTO group than the non-CTO group (Table I).
Table I
Periprocedural complications in the group of all-comers treated with PCI stratified by type of culprit lesion (CTO vs. non-CTO)
Patient characteristics stratified by CAP
Patients who experienced CAP were significantly older (p = 0.02), less often males (p = 0.004) and suffered more frequently from arterial hypertension (p = 0.01; Table II).
Table II
Clinical characteristics, coronary angiography and culprit lesion characteristics in patients treated with PCI according to the presence of CAP – CTO CAP vs. CTO non-CAP
[i] Data are presented as mean ± standard deviation or count (percentage); percentages reflect available study data. CABG – coronary artery bypass grafting,CAD – coronary artery disease, COPD – chronic obstructive pulmonary disease, CTO – chronic total occlusion, LAD – left anterior descending coronary artery, LMCA – left main coronary artery, MVD – multi-vessel disease, NSTEMI – non-ST segment elevation myocardial infarction, PCI – percutaneous coronary intervention, STEMI – ST segment elevation myocardial infarction, SVD – single-vessel disease.
Coronary angiography and culprit lesion characteristics
There were no statistically significant differences in the results of coronary angiography or culprit lesion characteristics between the CTO CAP and non-CAP CTO groups (Table II).
Procedural indices
Considering the type of PCI, significantly more patients in the CAP CTO group were treated without stent implantation, compared to the non-CAP CTO group (p = 0.005). These and other procedural indices are presented in Table III.
Table III
Procedural indices in patients treated with PCI according to the presence of CAP (CTO CAP vs. CTO non-CAP)
[i] Data are presented as mean ± standard deviation, median [interquartile range] or count (percentage); percentage reflects available study data. BMS – bare-metal stent, BRS – bioresorbable scaffold, CTO – chronic total occlusion, DCB – drug-coated balloon, DES – drug-eluting stent, FFR fractional flow reserve, IVUS – intravascular ultrasound, OCT – optical coherence tomography, PCI – percutaneous coronary intervention, POBA – plain-old balloon angioplasty, TIMI – thrombolysis in myocardial infarction.
Overall procedure-related complications
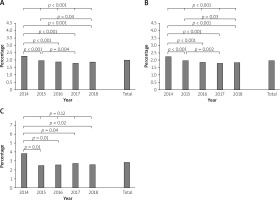
When assessing the overall group of patients treated with PCI, all procedural complication frequencies significantly decreased in the studied period of time (2.29% vs. 1.98% vs. 1.89% vs. 1.81% vs. 1.86%, p < 0.001; Figure 1 A). A similar trend was observed in the non-CTO PCI group (2.26% vs. 1.96% vs. 1.87% vs. 1.79% vs. 1.84%, p < 0.001; Figure 1 B). However, in the CTO PCI group, the frequency of procedure-related complications, with the exception of the year 2014, remained at a stable level and did not change significantly (3.84% vs. 2.49% vs. 2.56% vs. 2.73% vs. 2.58%, p = 0.12; Figure 1 C).
Coronary artery perforations
The frequency of CAPs in the overall group of patients treated with PCI during the assessed period demonstrated a statistically significant increase in consecutive years (0.15% vs. 0.17% vs. 0.19% vs. 0.16% vs. 0.2%, p = 0.02, Figure 2 A), whereas in the non-CTO PCI group of patients, the frequency of CAPs did not show a statistically significant change (0.14% vs. 0.16% vs. 0.17% vs. 0.15% vs. 0.18%, p = 0.1, Figure 2 B). Also, there were no statistically significant changes in the frequency of CAPs in the CTO PCI group, although it did tend to increase (0.5% vs. 0.64% vs. 0.78% vs. 0.74% vs. 0.85%, p = 0.18, Figure 2 C).
Predictors of all complications
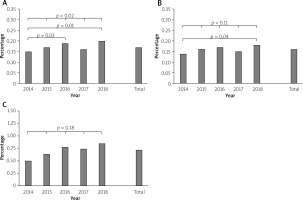
Before PSM, multifactorial logistic regression analysis performed in the overall group of patients treated with PCI revealed the following factors to be among significant predictors of all complication frequencies: age, diabetes mellitus, COPD, kidney disease, smoking, vascular access, prior PCI, MI, and stroke, patency of target coronary artery before PCI, type of PCI, type of culprit lesion and PCI within CTO culprit lesion (Figure 3 A).
Figure 3
A – Predictors of all periprocedural complications in the overall group of patients treated with PCI. B – Predictors of coronary artery perforation in the overall group of patients treated with PCI
CTO – chronic total occlusion, PCI – percutaneous coronary intervention, DES – drug-eluting stent, DCB – drug-coated balloon, POBA – plain-old balloon angioplasty, BRS – bioresorbable scaffold, BMS – bare-metal stent, TIMI – thrombolysis in myocardial infarction.

Predictors of coronary artery perforations
Prior to PSM, via multifactorial logistic regression analysis performed in the overall group of patients treated with PCI, the following factors were found to be significant predictors of CAPs: age, kidney disease, smoking, prior cerebral stroke, patency of target artery before PCI, no-reflow phenomenon, type of PCI and PCI within CTO culprit lesion (Figure 3 B).
Procedure-related complications stratified according to CTO PCI after PSM
After PSM, we extracted 5,652 patients treated within CTO and 5,652 patients with non-CTO PCI. Multifactorial analysis revealed that PCI within CTO was statistically significantly related to the higher frequency of CAPs (odds ratio (OR) = 1.89; 95% confidence interval (CI): 1.11–3.31, p = 0.01), while being less frequently correlated with deaths (OR = 0.44; 95% CI: 0.30–0.63, p < 0.001), no-reflow (OR = 0.69; 95% CI: 0.48–0.98, p = 0.03), dissections (OR = 0.13; 95% CI: 0.03–0.37, p < 0.001) and all complications (OR = 0.67; 95% CI: 0.55–0.82, p < 0.001).
Discussion
The main finding of the current study is that patients from the CTO PCI group presented significantly higher frequency of periprocedural complications and CAPs in comparison to the non-CTO PCI group. The frequency of overall periprocedural complications and CAPs remained stable in the CTO PCI group during the assessed period, while in the non-CTO PCI and all-comers groups, the overall periprocedural complication rate significantly decreased and the CAP rate significantly increased in the all-comers group. Patients from the CAP CTO group were significantly older, more often females, and more frequently treated from radial access in comparison to the non-CAP CTO group. Also, the effectiveness of PCIs in the CAP-CTO group, compared to the non-CAP CTO PCI group, assessed as patency of the coronary artery by TIMI grade scale after PCI, was significantly lower. Multifactorial regression analysis performed in the all-comers group of patients treated with PCI showed that PCI within CTO was related to higher CAP and lower overall complication rates. After PSM, CTO PCI was also significantly related to higher frequency of CAPs, and at the same time, lower frequency of deaths, no-reflow, dissections and overall periprocedural complication count.
The increase in CAP rate among the all-comers PCI group in consecutive years for the analysed registry, although remaining at a low level, is at least partially related to the different type of operators, when compared to other reported studies. Usually, the published registries include well-experienced operators, while in the present report among high volume PCI CTO operators, we also included beginners, which may explain the increase in the frequency of CAPs in recent years. The increase in beginner rate seems to be gradual for the last few years. It is also not without significance that patients qualified for PCI, including PCI CTO, present increasingly complex lesions, and despite the increasingly advanced equipment used in PCIs, there is a decreasing tendency in the total number of procedure-related complications, while the number of CAP remains at a constant level or even tends to increase depending on the group analysed in recent years. This was demonstrated in other registries published recently [15]. Low rates of CAPs in the assessed groups of patients, in comparison to other registries, could be mainly explained by underestimation, due to the way of reporting them. Reporting CAPs were left to the operator’s decision, while in other registries, additional core-lab analysis was performed, which delivers more precise results and usually elevates periprocedural complication rates.
Overall complications
The overall complication rate decreased in all of the observed groups of patients (CTO PCI, non-CTO PCI and all-comers PCI); however, interestingly, CTO PCI was found to be a negative predictor of overall complication rate in the all-comers group before and after PSM. Also, the overall periprocedural complication rate was higher among non-CTO PCI patients than in the CTO PCI group, which was mainly due to significantly higher rates of CAP, no-reflow and puncture-site bleeding.
It was reported in previously published studies that CAP, bleeding and contrast-induced nephropathy were more frequently observed in female patients [15, 16]. Female patients are under-represented in the published CTO PCI literature, with the proportion of female patients varying from 14% to 21%, while in our study, this totalled 25% [16]. The study published by Riley et al. demonstrated that the overall complication rate in CTO PCIs was 9.7% [1]. The most common adverse events were perforation (8.8%), MI (2.6%), arrhythmia requiring treatment (1.2%), cardiogenic shock (1.1%), and in-hospital deaths (0.9%). There were 9 procedure-related deaths (0.9%). All procedural deaths were associated with perforation and occurred among patients having undergone prior CABG surgery [1]. Independent predictors of complications during CTO PCI were: use of the retrograde approach, age and J-CTO score [1]. Prior analyses of CTO PCI complication rates were mainly based on a meta-analysis and data from the PROGRESS and RECHARGE registries. The meta-analysis reported a pooled complication rate of 3.1% among 18,061 cases, similar to rates in non-CTO PCI [11]. The PROGRESS registry demonstrated the overall periprocedural complication rates during hybrid CTO procedures of 2.8% from 1,569 cases. Complications reported included death (0.6%), MI (1.0%), emergent CABG (0.1%), stroke (0.3%), need for repeat PCI (0.3%) and tamponade from CAP requiring pericardiocentesis (1.0%) [17]. The RECHARGE is a similar contemporary European registry reporting an overall complication rate of 2.6%, with similar rates for death (0.2%), stroke (0.2%), MI (2.2%), major bleeding (1.9%) and tamponade (1.3%) [18]. In the study published by Riley et al., higher overall composite and constituent complication rates were reported compared to both of the other registries as well as ours. The authors explained the differences as to how CAP rates were reported, and concluded that their analysis also included mild perforations (stage I of the Ellis classification), which could be mistakenly over-reported due to different method of assessing them [1]. In the study published by Riley, this was counted by a core-lab team based on cine angiographies [1].
Independent predictors of procedural complications were similar in previously reported data [19]. Older age and any use of the retrograde approach were associated with increased procedural risk [20]. However, Xenogiannis et al. stated that in recent years, the rate of in-hospital MACE and mortality decreased in the group of patients treated with PCI within the CTO lesion and regarded in-hospital MACE (2% in the current era vs. 3% in the early era; p = 0.04), while for mortality decreased (0.2% in the current era vs. 0.7% in the early era; p = 0.04) [21]. This was attributed, among other factors, to the decrease in CTO lesion complexity, lower rate of retrograde crossing and antegrade dissection re-entry [21]. We do not possess such data for comparison. In contrast, patients undergoing CTO PCI were at more severe angina risk, while technical and procedural success rates remain high [21]. The mortality rate observed by Sapontis et al. was 0.9%, which is higher than previously reported in both hybrid and non-hybrid treated cohorts [19]. However, based on the data analysed in our analysis, even despite the PSM of the CTO PCI and non-CTO PCI groups, in the group of CTO PCI patients there were statistically significantly more patients with chronic coronary syndromes (37.8% vs. 30.8%), and fewer with unstable angina pectoris (30.0% vs. 32.3%), NSTEMI (19.2% vs. 17.4%) and STEMI (12.0% vs. 17.3%; p < 0.001). It resulted in a more severe average clinical condition of patients from the non-CTO PCI group at baseline, which was reflected in a higher percentage of cardiac arrests before admission to hospital (2.5% vs. 1.8%, p = 0.023).
Coronary artery perforations
The incidence of CAP in our cohort of patients was lower than that in a recent meta-analysis (2.9% to 4.3%) [11]. Also, compared to large real word registries, the frequency of CAPs was clearly lower in the analysis presented [15]. CTO PCI has been identified in previously published studies as a risk factor for CAP (OR: 3.96–14.7) [3, 9]. Other reported risk factors for CAP include older patient age, female gender, previous CABG and use of rotational as well as laser atherectomy [3, 9, 10, 16]. In the current analysis, we confirmed older age to be among the predictors of increased CAP frequency, apart from kidney disease, smoking, prior cerebral stroke, no-reflow phenomenon, type of PCI and culprit lesion as well as PCI within the CTO lesion. The strongest impact on the frequency of CAP was found for no-reflow, with the odds ratio of 6.382.
The study published by Parsh et al., which included an overall group of patients treated with PCI, revealed that patients with CAP were older, more likely to be female, have peripheral arterial disease or heart failure, require an intra-aortic balloon pump or other mechanical ventricular support prior to PCI, treatment of high complexity (type C) lesions, treatment of CTO and to be in cardiogenic shock at the beginning of the procedure compared to those without CAP. Conversely, patients with perforations were less likely to have diabetes [3]. Similar patient and angiographic characteristics as potential risk factors for CAP including older age, female gender, presence of chronic kidney disease, arterial hypertension, and previous PCI or CABG as well as angiographic characteristics such as type C lesions, CTOs, calcified lesions and culprit lesions in the right coronary artery, have also been identified in other studies [4]. Some of them were confirmed by our results. It was further noted that treatment of CTO was the strongest risk factor for CAP development [3]. In our study, no-reflow was the strongest, while CTO was also found to be a strong predictor. It was also demonstrated that the effect of CAP on mortality may be more deleterious in women than in men [11]. Among the several explanations of that relationship, the leading ones are the smaller vessel diameter and differences in vessel wall thickness in women. Azzalini et al., in their multi-centre study performed on 1,811 patients treated with PCI within CTO, found the CAP rate to be 5.5%. CAPs required intervention in half of the cases, and led to tamponade in 1/5 patients. Approximately half of the perforations were due to injury to the CTO vessel and the other half were related to the retrograde approach. The highest burden of acute morbidity and mortality observed in patients who developed CTO vessel-related perforations was related to age, long occlusions and more advanced techniques (rotational atherectomy, antegrade dissection/re-entry and retrograde approach) needing to cross complex occlusions [22]. Patients with perforation suffered an increased incidence of target vessel failure during the short-term follow-up [22]. In a very large study (n = 26,807) carried out by Kinnaird et al., it was found that CAPs may have an effect on mortality, with an odds ratio for 12-month, all-cause mortality of 1.60 for perforation survivors compared to matched subjects without CAP [7]. The authors attributed this to several possible explanations, such as incomplete revascularization due to procedure interruption secondary to perforation, concomitant complications (vascular complications, periprocedural MI, major bleeding, etc.), and restenosis/thrombosis of covered stents. As reported by Parsh et al., CTO PCI is the strongest independent predictor of developing CAP, being associated with a 7-fold increase in the adjusted risk of such a complication, compared to non-CTO PCI [3]. The aetiology of CAP in CTO PCI is multifaceted, and it can be related to the presence of severe calcification within the occlusion requiring aggressive plaque modification, proximal cap ambiguity and unclear vessel course, as well as the use of antegrade dissection/re-entry techniques and the retrograde approach, particularly through epicardial collaterals [23, 24].
Danek et al. reported in their study, performed on more than 2,049 patients treated with CTO, a CAP rate of 4.1%, and that CAP occurred more frequently in older patients and those with prior CABG [6]. They indicated the following to be among phenomena related to the higher rate of CAPs: prior PCI, RCA target vessel and angiographic complexity necessitating the use of advanced CTO crossing techniques [6]. Some of those factors were confirmed in our analysis. Sapontis et al., in a registry including 1,000 consecutive patients, reported that CAPs requiring treatment occurred in 4.8% of patients, and the overall perforation frequency was 8.8% [19]. When assessing type of the lesion, Sapontis et al. reported the frequency of de-novo lesions to be 89.3%, while the restenosis rate was 10.7%, and as reported in other studies, the right coronary artery was most commonly treated [19]. Similar percentages were observed in our registry with slightly lower frequencies of restenosis. The risks of CTO PCI are reported to decrease over time and be similar to those of non-CTO PCI [11]. Perforations were observed by the core-lab in 8.8% of cases and were reported by operators in 6.6% of cases. The authors explain that this higher perforation frequency may relate to the implementation of core-lab assessment and the tendency for operators to not report clinically insignificant perforations to avoid scrutiny [19].
Hirai et al., in a multi-centre registry including 1,000 consecutive patients treated with CTO PCI, reported an in-hospital death rate among patients with CAP of 10.1%, whereas in our registry, procedure-related death was reported in 1.1% of cases, while considering the CTO PCI group, this equalled 0.44%, and for the non-CTO PCI group 0.45%. Hirai et al. reported that in their study, 55.8% of patients with CAPs had a history of prior CABG, while in our study, this was only true in 7.7% of patients [25].
In another study published by Danek et al., a periprocedural complication rate of 2.8% was reported. In-hospital complications included: death, MI, recurrent symptoms requiring urgent repeat target vessel revascularization with PCI or CABG, tamponade requiring either pericardiocentesis or surgery and stroke [17]. It is clear that the study included only serious complications in comparison to our analysis. Danek et al. also confirmed older age as the most important predictor of complications [17]. This finding is consistent with our study and prior studies. It is likely related to more complex coronary anatomy with increasing age, higher prevalence of tortuosity, calcification, prior CABG and possibly, lower tolerance to inadvertent guidewire exits.
Limitations of our study include its retrospective, observational design, lack of core-laboratory adjudication, and limited follow-up. Relatively few coronary perforations were observed. There are several limitations to our study. First, our study was a retrospective analysis utilising data from a large database which does not include details on the severity of CAP (such as Ellis type classification) or vessel size, which would be valuable to examine when attempting to identify the aetiology of the potentially worse CAP prognosis in women than men. In addition, various outcomes that may develop after CAP, including tamponade, need for emergency surgery or subsequent development of MI, could not be evaluated in our population based on database restrictions. One of the key factors that may be significantly related to the incidence of CAP is the type of approach to CTO (antegrade vs. retrograde). Unfortunately, the database analysed by our team does not differentiate CTO procedures in this aspect and it is not possible to perform such an analysis, which eliminates the possibility of estimating one of the key factors that may affect the frequency of CAP occurrence and contributing to the bias analysis.
Conclusions
The main finding of the current study is that patients from the CTO PCI group presented significantly higher frequency of periprocedural complications and CAPs in comparison to the non-CTO PCI group. The frequency of overall periprocedural complications and CAPs has remained stable in the CTO PCI group in recent years, while in the all-comers group, CAP rates significantly increased during the same period. Patients from the CAP CTO group are significantly older, more often females and more frequently treated from radial access in comparison to the non-CAP CTO group. PCI within CTO performed in the all-comers group is related to a higher CAP rate. Also, after matching, CTO PCI was significantly related to a higher frequency of CAPs.