Introduction
Endometrial cancer (EC) is one of the most commonly diagnosed malignant gynecological tumors among females worldwide, and it is the most common malignant gynecological tumors in developed countries [1, 2]. There are two identified types of EC: type I tumors which are often preceded by endometrial hyperplasia, and type II tumors, which are derived from intraepithelial carcinoma, a precancerous lesion, and are predominantly serous carcinomas arising in atrophic endometrium [3]. Although there are many newly discovered treatments for EC, the prognosis of EC patients with advanced or recurrent EC is still unfavorable [4]. There is an urgent need to understand the genetic and molecular pathogenesis of EC oncogenesis. The forkhead box (FOX) proteins (which originate from a huge family of different transcriptional factors) play essential roles in various biological processes, such as cell proliferation, differentiation, and cell cycle regulation [5]. Forkhead box M1 (FOXM1) has a 100-amino-acid winged helix DNA binding domain and it is considered a novel anti-cancer target, because it has many important functions in mitosis regulation, cell cycle transition, as well as other carcinogenesis signaling pathways [6]. Nevertheless, the detailed data about its expression and cellular functions in EC remain unclear [7]. Dachshund homolog 1 (DACH1), a member of the Sno/Ski co-repressor family, forms a part of the retinal determination (RD) signaling pathway, it has a vital role in drosophila eye and limb development [8]. Interestingly, the expression of DACH1 was detected in many cancers and it was found to be associated with patients’ prognosis [9, 10]. Although DACH1 is hypothesized to be a tumor suppressor in EC tissue, its expression in EC pathogenesis remains vague. We decided to analyze FOXM1 and DACH1 expression, because both of them have been linked to many functions in mitosis regulation, cell cycle transition, and carcinogenesis signaling pathways, however, no previous study has assessed them together in EC. The aim of the study is to clarify the expression of FOXM1 and DACH1 in EC tissues and the relationship between their expression level, clinico-pathological features, and the prognosis of EC patients.
Patients and pathologic specimens
Our retrospective study was conducted on eighty formalin-fixed and paraffin-embedded biopsies, which were randomly selected from the archive of the Pathology Department, Zagazig University. The patients were operated on, diagnosed, treated, and followed up from February 2015 to February 2020. The hysterectomy specimens were retrieved: 50 patients with EC, 10 specimens of normal endometrium, and 20 specimens of endometrial hyperplasia. The endometrial hyperplasia specimens were divided into two sections: ten simple hyperplasia without atypia and ten with atypical hyperplasia. The normal endometria were obtained in two phases: five specimens in the proliferative endometrial phase and five specimens in the secretory endometrial phase. The clinico-pathological characteristics of our patients including demographic information, age, tumor size, tumor stage, pathological subtype, degree of myometrial invasion, cervical stromal invasion, adnexal and serosal invasion, parametrial extension, regional lymph node metastasis, peritoneal cytology, type of treatment received, follow-up data, and the patients’ outcomes were obtained from the patients’ medical records at the Clinical Oncology and Nuclear Medicine Department, Faculty of Medicine, Zagazig University.
According to patients’ files, all patients were operated on in the Department of Gynecology and Obstetrics, Faculty of Medicine, Zagazig University, whereas tissue specimens were diagnosed in the Pathology Department, Faculty of Medicine, Zagazig University. EC was graded and staged according to the International Federation of Gynecology and Obstetrics (FIGO) classification and the patients received radiotherapy and chemotherapy according to their staging at Clinical Oncology and Nuclear Medicine, Faculty of Medicine, Zagazig University.
All samples underwent processing and preparation to investigate FOXM1 and DACH1 expression changes in EC patients and non-neoplastic endometrial samples.
Immunohistochemistry method of staining and assessment of forkhead box M1 and dachshund homolog 1 expression
All tissue samples were immune-stained and incubated with primary mouse monoclonal anti-FOXM1 (ab207298) and primary rabbit polyclonal anti-DACH1 antibody (ab205718) dilution 1:1000 (Abcam, Cambridge, MA). The semi-quantitative scoring system was used to assess nuclear FOXM1, cytoplasmic DACH1 staining intensity, and extent of stain. Each specimen was assigned a score according to the intensity of nuclear FOXM1 staining and cytoplasmic DACH1 staining (no detected stain = 0), weak stain means slight yellow stain = 1, moderate stain means yellowish brown stain = 2, and strong stain means brown stain = 3. The extent of stained cells was scored as follows: 0% = 0, 1–24% = 1, 25–49% = 2, 50–74% = 3, and 75–100% = 4. The final stain score was obtained by multiplication of the two scores, it ranged from 0 to 12 taking 4 as a cut-off point to divide marker expression into high and low stain [7].
Statistical analysis
Categorical variables’ percentages were compared using the c2 test or Fisher’s exact test. The correlations between FOXM1 and DACH1 expression were determined using the phi coefficient (Φ). Overall survival (OS) was calculated as the time from the start of treatment to the date of patients’ death or last follow-up visit (censored). Progression free survival (PFS) is the duration from date of the start of treatment to the date of disease progression. Assessment of OS and PFS was done according to markers, they were estimated using the Kaplan-Meier plot method, and they were compared using the two-sided exact log-rank test. OS was done for all EC patients (50 patients). PFS was done for 25 EC patients (7 stage II patients with maximal stromal invasion, all stage III, and all stage IV). A p-value < 0.05 was considered significant. The statistics were performed using SPSS 22.0 for Windows and MedCalc for Windows.
Results
FOXM1 expression was detected in EC tissues more than normal endometrial tissues and more than endometrial hyperplasia tissues, these results were statistically significant (p = 0.001) and 0.01 respectively (Table 1, Fig. 1).
Fig. 1
Expression of forkhead box M1 in nuclei of endometrial carcinoma cells. A, B – high nuclear expression in high grade and stage endometroid endometrial carcinoma 400×, C – high nuclear expression in high grade and stage serous endometrial carcinoma 400×, D, E – low nuclear expression in low grade and stage endometroid endometrial carcinoma 400×, F, G – low nuclear expression in endometrial hyperplasia 400×
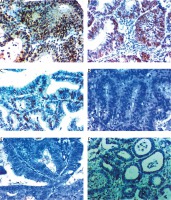
Table 1
Comparison between endometrial carcinoma and non-cancerous endometrial tissue regarding forkhead box M1 and dachshund homolog 1 expression
Forkhead box M1 expression and association with histopathological, clinical, and pathological findings in endometrial cancer samples
Levels of tissue protein expression of FOXM1 were positively associated with larger tumor size (p = 0.002), higher grade (p = 0.004), presence of lymph vascular invasion (LVI), myometrial invasion, cervical stromal invasion, adnexal invasion, positive peritoneal cytology, presence of lymph node metastases, higher FIGO stage (p < 0.001), presence of distant metastases (p = 0.029), and parametrial and serosal invasion (p = 0.003).
No significant association was found between FOXM1 and older age of the EC patients or histopathological subtype (Tables 2, 3).
Table 2
Clinicopathological features, immunohistochemical markers and outcome of 50 patients with endometrial carcinoma
Table 3
Relation between clinicopathological features and immunohistochemical staining for endometrial carcinoma patients N = 50
Patients with increased FOXM1 expression have higher incidence of tumor progression (p = 0.399) and poorer response to therapy (p < 0.001) in contrast to patients with low FOXM1 expression levels.
Kaplan-Meier survival analysis revealed that patients with higher expression of FOXM1 have worse 5-year PFS and 5-year OS rates than patients having lower FOXM1 expression levels (p = 0.26) and < 0.001 respectively (Tables 4, 5, Fig. 2).
Fig. 2
Expression of dachshund homolog 1 in the cytoplasm in cells of endometrial carcinoma. A – high cytoplasmic expression in low grade and stage endometroid endometrial carcinoma 400×, B – high cytoplasmic expression in endometrial hyperplasia 400×, C – low cytoplasmic expression in high grade and stage serous endometrial carcinoma 400×, D – low cytoplasmic expression in high grade and stage endometrial carcinoma 400×
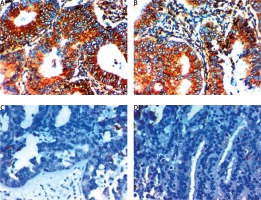
Table 4
Relation between immunohistochemical staining for forkhead box M1, dachshund homolog 1 and outcome in endometrial carcinoma patients (N = 50)
Table 5
Relation between immunohistochemical staining for forkhead box M1, dachshund homolog 1 and survival in endometrial carcinoma patients
DACH1 expression was lower in EC cells than normal endometrial tissue and endometrial hyperplasia, these results were statistically nonsignificant (p = 0.071) and 0.252 respectively (Table 1).
Dachshund homolog 1 expression and association with histopathological, clinical, and pathological findings in endometrial cancer samples
DACH1 expression was inversely associated with larger tumor size, presence of myometrial invasion (p = 0.002), older age of the EC patients, higher grade (p = 0.001), presence of LVI (p = 0.045), presence of distant metastases and adnexal invasion (p = 0.043), presence of lymph nodes metastases (p = 0.49), and higher FIGO stage (p = 0.022).
No significant associations were found between DACH1, serosal invasion, parametrial invasion, cervical stromal invasion, positive peritoneal cytology, or histopathological subtype (Table 2, Figs. 4, 5).
Fig. 3
A – Kaplan-Meier survival curves illustrating overall survival of the studied group, B – differences in patients as regards forkhead box M1, C – differences in patients as regards DACH2
FOXM1 – forkhead box M1
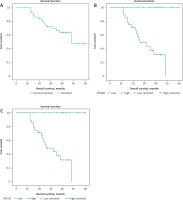
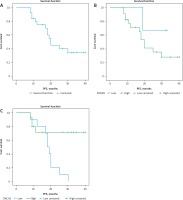
Fig. 4
A – Kaplan-Meier survival curves illustrating progression-free survival of the studied group, B – differences in patients as regards forkhead box M1, C – differences in patients as regards DACH2
PFS – progression-free survival, FOXM1 – forkhead box M1
Patients with increased DACH1 expression have lower incidence of tumor progression (p = 0.001), lower mortality (p = 0.026), and better response to therapy (p = 0.003) in contrast to patients with low DACH1 expression levels.
5-year PFS and 5-year OS rates were higher in patients having elevated DACH1 protein than those having suppressed DACH1 expression (p = 0.013) and 0.017 respectively) (Tables 4, 5).
We found an inverse association between expression of FOXM1 and DACH1 in EC tissues and non-neoplastic endometrial tissues (Φ–0.250), p = 0.007.
Discussion
The histological factors could affect EC prognosis such as clinical stage, depth of myometrial invasion, lymph vascular space invasion (LVSI), and histological subtype [7]. However, it was found that EC patients with the same clinical and pathological prognostic features had variable outcomes. So, specific prognostic biomarkers associated with clinical features and prognosis of EC were needed. We found that FOXM1 is over-expressed in tissues of EC and its high expression level was related to poorer prognosis of EC patients, unfavorable patients’ outcomes, and undesirable prognostic parameters. Our findings are consistent with results of Hu et al. [6] and results of Feng et al. [7], who reported a similar association between FOXM1 expression and dismal patients’ outcome in hepatocellular carcinoma and EC. Previous studies indicated that FOXM1 is an essential regulator of a plethora of biological processes, and its dysregulation could contribute to oncogenesis and cancer progression [11]. FOXM1 expression is considered a significant predictor of dismal clinical outcome in patients with many solid tumors [12, 13], and it regulates many proliferative signals in the cell cycle [5]. If FOXM1 (a transcription factor) is over-expressed, it is considered a potential therapeutic target in a wide range of human cancers [14]. Moreover, FOXM1 is involved in oncogenesis, epithelial-mesenchymal transition (EMT), malignant cell invasion, and metastasis [15]. Similar results were reported by Wen et al. [16], who stated that high FOXM1 expression in ovarian cancer patients is related to an unfavorable outcome as it can promote ovarian cancer cell proliferation, invasion, and metastasis. In addition, FOXM1 plays a vital role in progression of esophageal cancer, as it can increase the expression of MMP-2 and MMP-9, which leads to promoting malignant cell invasion and spread [17]. Furthermore, FOXM1 can promote angiogenesis in several cancers through enhancing vascular endothelial growth factor expression, so it promotes cancer progression [18, 19]. Feng et al. [7] found a reduction in proliferative and invasive ability of cells of EC after knocking down FOXM1, which proved the impact of FOXM1 in progression of EC and its role as a new prognostic biomarker for EC.
Another important impact of FOXM1 in EC progression is its role in resistance to chemotherapy that was increasingly identified [6].
Based on the plethora of identified impacts of FOXM1 in many cancers, further studies are needed to clarify its relevance to EC progression. Due to the lack of detailed studies about mechanisms of action of FOXM1 in EC, we assessed the expression of another marker, DACH1.
We found that DACH1 is down-regulated in EC tissues in comparison to normal endometrium, and its expression was associated with a favorable outcome and better pathological parameters. Previous studies showed very similar results to ours: in EC, squamous cell carcinoma cells, and in renal carcinoma tissues [2, 20].
Zhou et al. [2] noted that DACH1 is down-regulated in EC tissues compared to non-malignant endometrium and is positively associated with better outcome.
Zhang et al. [20] reported that the expression of DACH1 was more down-regulated in squamous cell carcinoma cells than adjacent non-neoplastic mucosa, and reduction of its expression was found in the cancers that spread to lymph nodes and distant organs. Moreover, up-regulation of DACH1 expression led to suppression of malignant cell proliferation, invasion and spread, and evoked induction of apoptosis in malignant cells. Therefore, DACH1 can be considered one of the novel therapeutic targets which is expected to improve EC patients’ prognosis.
Chu et al. [21] observed reduced expression of DACH1 in renal carcinoma tissues, it was inversely associated with tumor cell proliferation, grade, and TNM stage. Additionally, they demonstrated that DACH1 function restoration in renal clear cell cancer cells could lead to inhibition of tumor growth.
DACH1 expression leads to down-regulation of transcription of cyclin D1, which is the key in controlling malignant cell proliferation, so, DACH1 re-activation represents a potential therapeutic target of cancer.
Recently, it was found that DACH1 inhibition leads to stimulation of oncogene dependent cancer cell proliferation, invasion, and migration, which was established in breast cancer cells [9, 10]. Our results demonstrated that over-expression of DACH1 leads to suppression of proliferation and metastatic ability of EC cells. Similar results were observed in cells of gastric cancer, where there is marked reduction in DACH1 expression in chemo-resistant cancers compared with chemo-sensitive cancer, consequently, it could be a predictor for chemo-resistance in malignant cells [22]. Overall, all these previous results and our results suggest that DACH1 leads to suppression of tumor progression. Studies have confirmed that DACH1 expression leads to inhibition of SNAI1 and TGF-β-, thus, it inhibits EMT, which includes mechanisms that are involved in breast cancer cell invasion and metastases. Consequently, this adds to our results indicating that DACH1 leads to regulation of EMT in EC [23, 24]. Zhou et al. [2] observed that up-regulation of invasion and metastasis markers (N-cadherin and vimentin) and down-regulation of epithelial markers (E-cadherin and β-catenin) in DACH1 negative cancer cells indicate a rise of cancer cell invasion and spread. On the other hand, up-regulation of DACH1 resulted in increased expression of E-cadherin and β-catenin, these observations suggested that increased DACH1 expression might be able to inhibit EMT by inhibition of the Notch1 pathway through c-Jun. Nevertheless, over-expression of DACH1 decreased cyclin D, c-Jun, Notch1, and Hes1 expression. All these agents vastly promote cancer progression, so knocking down DACH1 leads to down-regulation of these proteins.
Our data showed that FOXM1 leads to EMT induction in EC cells in contrast to DACH1, we found a negative correlation between them in both EC and non-malignant endometrial tissues. FOXM1 and DACH1 influence cancers of various organs, and this is the first study to assess their expression together in EC and show the association with patients’ prognosis. Both FOXM1 and DACH1 can affect EC patients’ prognosis, but their underlying mechanism remains unclear. Further studies are needed to understand the molecular mechanism between them and EC prognosis, which will be used to design better intervention strategies to predict and improve prognosis of EC patients.
Conclusions
Our study identified FOXM1 over-expression in addition to down-regulation of DACH1 in EC compared to normal tissues. Furthermore, we explained the positive relationship between their expression and clinical, pathological data, and patients’ prognosis. We found an inverse association between FOXM1 and DACH1, therefore, all of our data suggest that FOXM1 and DACH1 can be considered as attractive therapeutic targets and prognostic biomarkers for EC and possibly for other cancers which are characterized by FOXM1 over-expression and/or down-regulation of DACH1 expression.







