Introduction
Nowadays, as it was postulated in the 2021 ESC/EACTS Guidelines for the management of valvular heart disease, transcatheter aortic valve replacement (TAVR) has become a new standard of care for patients with aortic stenosis who are not suitable candidates for conventional surgery [1]. The number and complexity of TAVR procedures continue to grow, but there are limited data on the indications, procedural characteristics, complications, and outcomes in very high-risk patients, supported with prophylactic veno-arterial extracorporeal membrane oxygenation (V-A ECMO).
Aim
In the present study, we analyzed the 4-year survival in TAVR patients who were operated on using a written algorithm for prophylactic awake peripheral V-A ECMO at the reference heart surgery and ECMO center in Kazakhstan.
Material and methods
From June 2012 to October 2022, 590 consecutive patients underwent TAVR at our center. Of these, 27 (4.5%) patients underwent TAVR with prophylactic V-A ECMO because they were deemed very high risk for periprocedural complications and formed the study population (Table I). The study was conducted in accordance with the Declaration of Helsinki, and approved by the Local Bioethics Committee of the National Research Cardiac Surgery Center (No. 01-74/2021 from 6 October 2020). Informed consent was obtained from all subjects involved in the study. All cases were discussed in multidisciplinary heart team meetings. The indications for prophylactic V-A ECMO were based on the established standardized protocol for the utilization of ECMO at our center, and included depressed left ventricular ejection fraction (< 35%), severe pulmonary artery hypertension, vasopressor and/or inotropes requirement prior to TAVR, and decompensated or high-grade heart failure [2].
Table I
Baseline clinical details
Prophylactic V-A ECMO implantation (Stockert, Sorin Group Deutschland GMBH, München, Germany, and Deltasrtream MDC, Medos/Xenios, Xenios AG, Heilbronn, Germany) was performed via surgically assisted transfemoral approaches under monitored conscious sedation and local anaesthesia. The veno-arterial cannulation was performed via a mini cut-down open technique. Arterial and venous cannulae (Medtronic Bio-Medicus, Tijuana, Baja, California, Mexico) were selected according to the patient’s biometric parameters. Blood was drained from the right atrium and the inferior vena cava, oxygenated and decarboxylated in the ECMO device and returned to the iliac artery. In all cases the prophylactic V-A ECMO was initiated in the operating theater prior to TAVR. The ECMO settings at the start of the procedure were as follows: priming with 800 ml of balanced electrolyte isotonic solution (Sterofundin, B. Braun Melsungen, AG, Germany). The initial pump speed was 1 l/min. After vascular access was achieved, the circuit was connected to cannulae and flow was initiated at a low flow rate, then increased incrementally to the target rate over a short time. Gas flow rates were set in relation to blood flow. The ECMO flow with a minimum of 1200 ml/min and gas sweep rates were adjusted in each case to maintain target mean arterial pressure ≥ 70 mm Hg and normocapnia, to maintain spontaneous breathing. During rapid pacing for balloon valvuloplasty, deployment and post-dilatation of the transcatheter heart valve, the pump was running at stable speed.
TAVR valves used included balloon-expandable Sapien XT (Edwards Lifesciences, Irvine, CA, USA), Myval (Meril Life Sciences Pvt. Ltd., Vapi, Gujarat, India), and self-expandable EvolutR (Medtronic, Irvine, CA, USA).
Complications
ECMO-related vascular or bleeding complications that were followed were: limb ischemia, vascular injury (dissection, pseudoaneurysm formation, retroperitoneal bleeding), need for blood transfusion, and groin infection.
TAVR-related complications that were followed were: complete atrio-ventricular block, prosthetic valve embolization, need for the second valve implantation, aortic annulus rupture, aortic wall rupture or dissection.
Results
During the hospital stay, there were no prophylactic V-A ECMO associated, hemodynamic, or TAVR procedural complications.
The process of weaning and decannulation of the ECMO system was performed in 92.6% of cases at the end of the procedure in the operating theatre. Two patients underwent this process in the ICU under monitored conscious sedation. The mean duration of prophylactic V-A ECMO for procedure support was 51.4 ±10.3 min. There were no ECMO-related vascular or bleeding complications (Table II). Device success was achieved in all cases.
Table II
Periprocedural characteristics of prophylactic awake peripheral veno-arterial ECMO
We observed 74% survival through a 4-year period of follow-up (Figure 1 A). During this period, 4 (14.8%) patients died within 4 months after intervention. Two other patients died in 20 and 22 months after TAVR, respectively. The causes of death are unknown except in the case of 1 female patient, who died 20 months after the operation due to ketoacidotic coma.
Figure 1
Kaplan-Meier graph for survival (A) and functional class by New-York Heart Association classification changes (B) at 4-year follow-up
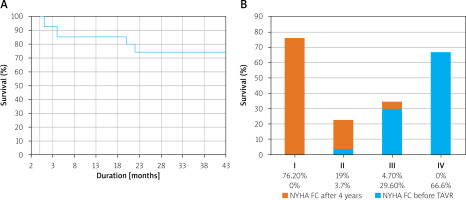
Functional class by New York Heart Association classification at 4-year follow-up changed dramatically in almost all survived patients (Figure 1 B).
Discussion
According to current guidelines, prophylactic veno-arterial extracorporeal membrane oxygenation may be a feasible procedural hemodynamic support in patients with aortic stenosis who present a high perioperative risk [3]. However, experience with its use during high-risk TAVR operations is limited. Data published to date support a promising clinical outcome in terms of procedural safety and survival in this highly vulnerable patient category [4–6]. Unfortunately, data on the prophylactic use of ECMO for TAVR are limited due to small sample sizes. Therefore, the influence on outcomes remains unclear.
The only study with a large number of cases investigating the impact of prophylactic V-A ECMO in patients with depressed left ventricular ejection fraction undergoing transcatheter aortic valve implantation was performed by Trenkwalder et al. [7]. This study did not find that periprocedural prophylactic ECMO support improved outcomes. The evidence suggesting potential harm of prophylactic V-A ECMO in patients undergoing TAVR needs to be clarified in future studies. Indeed, the essence of V-A ECMO is a hemodynamic bridge to support the patient, and to improve the safety and efficacy of TAVR.
In the systematic review of the early experience of TAVR, periprocedural CPB or VA-ECMO was used in 4%, as an emergency support for procedural complications. Short-term mortality was 29.8% and 1-year mortality was 52.4% in TAVR patients requiring short-term circulatory support [8]. At this point, two major concerns arise: whether we should discuss the impact of a prophylactic (not salvage) ECMO bridge on long-term outcomes, and the underlying extent of cardiac damage associated with valvular disease and its important prognostic implications for clinical outcomes after TAVR. Moreover, transcatheter technology nowadays serves a wide valvular pathology spectrum, which may challenge the patient’s clinical pathway and surgical options to be bridged by ECMO.
Survival after TAVR on ECMO is multifactorial. It is conceivable that the difference starts with the hospital course. Raffa et al. [9] published data from the ECMO in TAVR Investigators Group. The overall survival of patients requiring V-A ECMO during TAVR was 73% (74 patients out of 102). In cases of emergency V-A ECMO support, the in-hospital survival rate was 61% (40 patients out of 66), whereas the survival rate was 100% in the prophylactic implantation group. Major periprocedural complications such as bleeding, vascular injury, tamponade, stroke, and acute kidney failure can occur in up to 7.6% of TAVR procedures having prognostic implications [10]. Interestingly, we did not detect any ECMO-related vascular complication or ECMO-related life-threatening bleeding. This may be explained by the rather short time of ECMO support and the elective cannulation prior to the procedure as compared to emergency indications.
This emphasizes the importance of an experienced heart team including interventional cardiologists, cardiac surgeons, anesthesiologists and a perfusionist in specialized heart valve centers with written standardized protocols for the utilization of ECMO [10].
In our institution, we prefer to use the mini cut-down technique to expose femoral vessels for many reasons. First, this access provides straight, adequate exposure of the common femoral artery in all patients, including obese ones. Second, the incision runs parallel to the stress lines, reducing wound tension, and promoting healing. It prevents cutting across the inguinal creases and reduces the potential source of wound dehiscence and infection. Third, the open, meticulous technique of artery and vein restoration excludes false aneurism formation, major hemorrhage, and distal malperfusion. In addition, we have found the vessel cannulation through counter-aperture incision and subcutaneous tunneling using the Seldinger technique very safe due to damping mechanical forces at the tunnel level. Moreover, this strategy is preferable in the case of prolongation of ECMO for some days after TAVR, potential need for distal leg perfusion line insertion, and subsequent decannulation.
There are few studies in the literature reporting long-term survival using prophylactic support with V-A ECMO. Published papers [4, 6] reported that preemptive use of ECMO in selected high-risk patients was associated with 100% procedural success and 30-day survival of 100%. Periprocedural complications were comparable to the standard TAVR cohort, suggesting planned ECMO to be a feasible support in high-risk procedures that may otherwise have been declined [11].
Myocardial contractile reserve is another debatable factor and a subject of controversies in the literature. Worse outcomes in patients lacking contractile reserve were found in a retrospective analysis of patients undergoing the dobutamine stress echocardiography test prior to TAVR [12]. Généreux et al. concluded that the extent of cardiac damage is one of the strongest independent predictors of 1-year mortality after aortic valve replacement (AVR), underscoring the persisting detrimental impact of extravalvular consequences of AS despite AVR [13]. However, Ribeiro et al. reported that the absence of contractile reserve before intervention did not result in worse outcomes [14].
The strengths of this study include the established institutional standardized protocol guiding decision making regarding ECMO support for the individual patient [2], and 4-year, complete follow-up.
Limitations. The small number of patients represents the major limitation of our study. It is clear that large randomized trials are needed to address the feasibility and safety of prophylactic V-A ECMO for high risk TAVR. The second limitation is the absence of a control group. We expect to overcome this limitation with the accumulation of more experience in larger clinical series. The third limitation was the problem of identifying the exact cause of death of operated patients in the long-term period, which led to a misunderstanding of the initial trigger cascade of lethal consequences.
Conclusions
Among patients who underwent prophylactic V-A ECMO during very high-risk TAVR, the survival rate within 4 years was 74%, with low functional class of heart failure. This follow-up data support the feasibility of the established standardized protocol for the utilization of prophylactic V-A ECMO.








