Introduction
Acute abdomen can be caused by several gynaecological conditions in postmenopausal women, including annexal torsion, pelvic abscess, pyosalpinx, and haemoperitoneum due to rupture of annex masses. Additional causes are twisted uterine myomas and concomitant use of anticoagulant therapy or of hormonal replacement therapy with retrograde bleeding due to the stenotic cervix [1–6]. Acute abdomen with haemoperitoneum is a common gynaecological emergency but is rare in postmenopausal women. The diagnosis is more difficult to make in postmenopausal women as compared to those in reproductive age [1, 7]. According to literature, the rupture of a granulosa cell tumour (GCT) of the ovary is a rare but possible reason of haemoperitoneum due to the high vascularity of the mass, with an estimated rate of 10% [8, 9] to 15–17.6% [1, 10–12].
Described for the first time by Rokitansky in 1855 [12], GCT of the ovary accounts for 70% of all sex-cord-stromal tumours and is characterized by a low grade of malignancy. According to clinical presentation and histological features, GCT is divided into juvenile and adult variants. The juvenile form is predominantly in children and young women (< 30 years), and the adult form (90% of GCT) is primarily seen in early postmenopausal women. Granulosa cell tumours are oestrogen-producing tumours, but a small subset is androgenic. Common symptoms in juvenile cases are menstrual complaints, amenorrhoea, excessive bleeding, infertility, breast tenderness, and peripheral precocious puberty in paediatric populations. Androgen secretion may induce virilizing symptoms [13, 14]. In older patients the symptoms of GCT may mimic those of common epithelial ovarian cancer, such as vague abdominal discomfort, increasing abdominal girth, and weight loss [10], or they can appear suddenly with acute abdomen due to torsion or rupture of the mass with haemoperitoneum [15]. Postmenopausal women can present with vaginal bleeding (10%) due to endometrial hyperplasia or a well-differentiated endometrial cancer [16]. The onset of breast cancer because of hyperoestrogenism occurs in this population. Ascites appears in 10% of patients [10], and Meigs syndrome is also possible [17].
Patients might also report a reduction in vasomotor symptoms because of suppressed FSH by high oestrogen levels [7]. In uncertain cases testing for FOXL2 mutation can help with diagnosis, as 97% of adult GCT showed mutation of this gene [18].
Through ultrasound, most GCTs appear as large multilocular-solid masses with many locules, or as solid tumours with heterogeneous echogenicity of the solid tissue. Haemorrhagic components are common, and increased vascularity is demonstrated in colour or power Doppler ultrasound examination [19].
The gold standard treatment is surgery. In patients of reproductive age with disease International Federation of Gynaecology and Obstetrics (FIGO) stage I, it is possible to proceed with a fertility-sparing approach through unilateral salpingo-oophorectomy and peritoneal staging. In postmenopausal women and in those with more advanced disease, the most appropriate surgical treatment is a total abdominal hysterectomy with bilateral salpingo-oophorectomy, multiple peritoneal biopsies, and removal of all visible disease [12, 18, 20].
The prognosis for women with GCT is good. Although rare, metastasis may occur many years after the diagnosis [21]. Most of the diagnoses are at stage I disease (80–90%) and will therefore be expected to have a favourable outcome. Some authors describe a 5-year survival rate of more than 90%, but the presence of extraovarian disease correlates to a lower 5-year survival rate (33–50%) [20]. There is still debate about the negative prognostic factors that predict poorer outcomes for this disease because of the relative rarity of these tumours and the long follow-up required to study them. As reported by some authors [8, 22, 23], the stage of disease is the only prognostic factor associated with the risk of relapse. Tumour rupture, tumour size, and cellular atypia are other factors of prognostic significance [10, 12, 24, 25].
With this article we present a case report of a 53-year-old post-menopausal woman with acute onset of haemoperitoneum due to GCT rupture. We also present a narrative review to highlight the best management of this condition and to raise awareness of this unique clinical presentation.
Case presentation
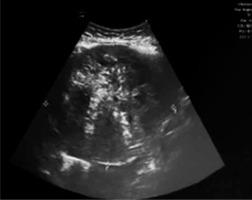
In June 2019 a 53-year-old Caucasian woman presented to the emergency department of “San Tommaseo dei Battuti” Hospital, Portoguraro (Veneto, Italy) complaining of severe abdominal pain, mostly suprapubic, and nausea for the previous 12 hours. There was no vaginal discharge, and the woman reported regular bowel movements and normal micturition. She has been in good general health prior to this incident, with 2 previous vaginal deliveries. She noted 2 years of physiological menopause. Past medical, surgical, family, and social histories were non-contributory. Clinical examination showed stable vital signs, a distended-painful abdomen especially in hypogastrium, diffuse tenderness, dullness to percussion, and positive for both rebound and guarding. Bimanual examination revealed a palpable, tender, and painful mass extending from the pelvis and reaching the umbilical transverse line. Pelvic ultrasound (Fig. 1) showed a non-retroverted uterus with regular structure, a thin endometrium, and a pelvic inhomogeneous mass with 12 × 11 × 12 cm maximum diameters, characterized by irregular margins and low vascularization (colour score 2) without a clearly discernible origin. Annexes were not visualized. Moderate free fluid was identified as occupying the Douglas’ pouch, the perihepatic, and the peri splenic zone. Abdominal ultrasound confirmed the pelvic mass and free fluid localized at the Morison’s pouch and at the perisplenic spaces. Computed tomography showed a solid pelvic mass with maximum diameters of 12 × 9 × 15 cm and inhomogeneous density compatible with annexal or ovarian lesion as well as free fluid in the abdomen and pelvis. The liver, spleen, pancreas, and kidneys were normal. Initial haemoglobin was 11.7 g/dl, platelets count 126 × 103/µl, C-reactive protein 1.10 mg/dl; coagulations tests, and renal and liver functions were regular. ECG was normal. Resuscitation fluids were soon administered intravenously.
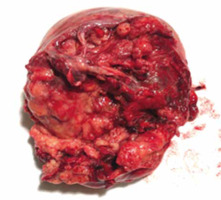
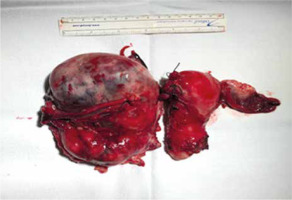
Given the rapid clinical worsening accompanied by haemoglobin fall at 9.5 g/dl, an urgent laparotomy was performed. Exploration of the abdominal cavity revealed haemoperitoneum and free blood clots. The right ovary was subverted by a bilobated necrotic- haemorrhagic mass of 15 cm. The cystic capsule was smooth on its anterior side but absent in the posterior side, which seemed to be the source of the bleeding. The mass was adherent to the right vaginal angle and the external iliac artery. The uterus and the left fallopian tube with the homolateral ovary appeared normal. Suspecting malignancy, an exploration of the abdomen was performed. There was no bowel or omental disease. Hepatic Glissonian and paracolic showers were free from macroscopic lesions. Surgeons performed a right salpingo-oophorectomy with excision of the pelvic mass (Fig. 2), total abdominal hysterectomy, left salpingo-oophorectomy (Fig. 3), and omental biopsy. Due to the surgical risk of damaging the external iliac artery, the surgeons left a 0.5 cm margin of adjacent residual tissue localized at the right vaginal angle. The extemporaneous examination of the mass was not performed because it was not available at our hospital. Intraoperative markers (Ca 125, CEA, Ca 19.9, Ca 15.3, a1 FP, inhibin B) were negative. Final blood loss was approximately 1600 cc. During surgery, the patient was transfused with 3 units of packed red cells.
The patient’s postoperative care was uncomplicated, and she was discharged 6 days after admission.
The definitive histological diagnosis was a GCT to the right ovary with extensive necrosis and haemorrhage. Uterus, left annex, and omentum were negative for malignancy.
After surgery the patient underwent a 6-course regimen of systemic adjuvant chemotherapy with carboplatin and paclitaxel. She started a subsequent regular follow-up without relapse.
Material and methods
We present a case of gynaecological acute abdomen with haemoperitoneum caused by the rupture of a GCT in a menopausal adult; the GCT was not previously diagnosed. We also conducted a systematic review of the literature by consulting multiple bibliographic databases research until November 2021. PubMed, Google Scholar, Cochrane Central Register of Controlled Trials databases and Ovid were queried to identify all articles regarding cases of haemoperitoneum in menopausal women with a GCT rupture. We also collected additional relevant articles from the reference lists of the primary identified studies. All authors participated in the search strategy and in the definition of the inclusion and exclusion criteria. The research strategy included different combinations of the following terms: haemoperitoneum granulosa menopause – spontaneous haemoperitoneum – granulosa cell tumour menopause – granulosa cell tumour ovary – case report. Pertinent articles were carefully evaluated. Full manuscripts were obtained for selected articles, and the decision for final inclusion was made after a detailed examination of the paper. We only included studies written in English. We excluded articles in press, books, conference abstracts not supported by full-text articles, and unpublished accepted manuscripts. We found case reports, retrospective analyses, and reviews of the literature. Duplicates were identified by an independent manual screening performed by one researcher and then removed. We followed the PRISMA guidelines [26, 27]. The systematic review was not submitted to Prospero because only a limited number of case reports were found in the literature. The patient provided informed consent for the publication and the use of her images.
Results
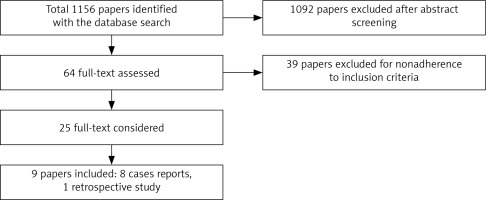
The literature research, conducted until November 2021, identified a total of 1156 articles. After initial screening we assessed 64 full-text papers of which 39 did not adhere to the inclusion criteria. Finally, 9 articles were included: 8 case reports and one retrospective study (Fig. 4). A total of 11 patients were analysed in this review, including the present case report. The first case was described in 1948 [13] and the last one in 2019 [28].
Fig. 4
Flow diagram according to Preferred Reporting Items for Systematic Reviews and Meta-analyses statement of the literature search algorithm
All cases were treated with primary surgery, with radicality depending on the clinical status of the patients. Only one case, which presented in shock state, was initially treated with embolization of the left uterine artery. The following day, she underwent open biopsy with frozen section followed by total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and bilateral iliac lymph node sampling [28].
The clinical characteristics of the patients and the histopathological elements of the tumour are summarized in Table 1. The mean age of the patients was 60.8 years. Most of the women had parity of more than 2 (7/11, 63.6%). For 3 women parity was unknown (27.2%). All women had a physiological menopause. Granulosa cell tumour was more frequent on the left ovary (6/11, 54.5%) than on the right one (45.4%). The tumour diameter was available for 10 patients, and the mean diameter was 10.1 cm (range 4–15 cm). The most common presentation associated with abdominal pain was vaginal bleeding (45.4%), of which 3 were associated with endometrial hyperplasia (27%), one with endometrial cancer (9%), and one with a normal endometrium despite the bleeding (9%). In summary, we found 5 cases with endometrial pathology (45.4%) of which 4 (36%) were associated with postmenopausal bleeding. Three patients had no reported endometrial status at histological examination. There were no reports of ascites. The histologic examination of the mass was available for all cases, of which 5 cases reported the subtype “Adult” (45.5%).
Table 1
Distribution of clinical characteristics of the patients and histopathological elements of the mass
| Case (n°) | Authors | Years | Age at menopause | Parity | Laterality | Mass size (cm) | Symptoms associated at abdominal pain | Endometrial status | Histology |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Present case (2019) | 53 | 51 | 2002 | Right | 15 × 12 × 11 | Nausea | Normal | Adult GCT |
| 2 | Brewer et al. (1948) [13] | 48 | 44 | 2012 miscarriage | Right | 9 × 7 × 5 | Asthenia | NA | GCT |
| 3 | Tufan et al. (2012) [1] | 54 | 52 | 2012 | Right | 13 × 12 × 8 | Postmenopausal bleeding, abdominal distension | Complex hyperplasia | Adult GCT |
| 4 | Scully et al. (1995) [11] | 56 | 50 | 0040 | Left | 11 × 7 × 2 | Anaemia, syncope, pain hemithorax right | Normal | Adult GCT |
| 5 | Poma et al. (1998) [2] | 76 | 63 | 2002 | Left | 10 × 9 × 3.6 | Anorexia, nausea, diaphoresis | NA | GCT |
| 6 | Ukah et al. (2011) [16] | 65 | 59 | 10 live births | Left | 5 × 5 | Vaginal bleeding | Endometrial cancer endometrioid well-differentiated | Adult GCT |
| 7 | Borah et al. (2010) [7] | 70 | 55 | 8008 | Right | 12 × 11 × 7 | Vaginal bleeding, lump abdomen | Cystoglandular hyperplasia | GCT |
| 8 | Genton (1980) [29] | 60 | NA | NA | Left | 10 | NA | Cystoglandular hyperplasia | GCT |
| 9 | Genton (1980) [29] | 71 | NA | NA | Left | 12 × 7 × 6 | Vaginal bleeding | Cystoglandular hyperplasia | GCT |
| 10 | Alhendi et al. (2019) [28] | 56 | NA | NA | Left | NA | Shock | NA | Adult GCT |
| 11 | Kakani et al. (2012) [25] | 60 | 44 | 4004 | Right | 4 × 4 | Vaginal bleeding | Normal | GCT |
Discussion
The present review shows that the research dedicated to GCT is poor given the low incidence and the difficulty in diagnosing the disease.
According to the literature, oestrogen secretion from GCT in post-menopausal women is responsible for endometrial pathology with a rate that varies 26–76% [12]. In detail, the literature reports endometrial hyperplasia in 32–85% of cases and endometrial cancer in 3–22% of cases [14]. In our review, we found endometrial pathology in 45% of the cases: 3 cases of hyperplasia (27%) and one case of cancer (9%).
In our case report, the surgeons decided to dose tumoural markers during surgery because they suspected a malignant mass. Only one paper [11] reported dosing LDH that turned out to be high, probably due to the patients’ haemolysis described in the same case report.
An interesting finding to consider is that the presentation of GCT is not always in the form of overt endocrine disturbance but can onset with acute abdomen because of rupture of a cystic compartment with haemoperitoneum. Granulosa cell tumour should remain in the differential diagnosis in all patients with abdominal pain and imaging suspicious for a gynaecological malignancy or pelvic mass of unclear origin, without ascites. Clinicians should be aware of GCT occurring in menopause, and that they are prone to rupture in about 10–15% of cases. Open biopsy frozen section is useful to avoid incomplete surgeries, especially when performed in postmenopausal women in urgent conditions.
Our study is strengthened by the long period of literature reviewed. All studies selected during the eligibility phase were further evaluated by manual comparison of populations, study settings, and authors to avoid overlapping cases.
The limitations of our study were represented by the small number of cases described in the literature and included in our review, due to the low incidence of sex cord stromal tumour (2–5% of all ovarian cancer)
And the absence of complete anamnesis of the patients, such as past medical, surgical, family, and social histories. Our review was also influenced by the lack of a comparable histopathological description of the mass. The histological diagnosis was not available for all papers, but the median age of the women met the criteria for the adult subtype. Furthermore, our work was limited by the lack of pre- or intraoperative tumour markers, particularly inhibin B.
Conclusions
We recommend that clinicians retain GCT in the differential diagnosis of all patients with abdominal pain and imaging indicative of a gynaecological malignancy or pelvic mass. Open biopsy frozen section is useful for the execution of adequate and radical surgery, especially when performed in a postmenopausal woman presenting with an abdominal mass associated with vaginal discharge.