The Fontan procedure is a milestone in paediatric cardiac surgery designed to treat complex single-ventricle congenital heart defects by rerouting systemic venous blood directly to the pulmonary arteries, bypassing the ventricle [1, 2]. While this procedure improves systemic oxygenation, it creates non-physiological haemodynamics over time. Long-term Fontan circulation is associated with elevated central venous pressure, increased pulmonary resistance, and low cardiac output, often leading to circulatory failure. Common complications include liver dysfunction, arrhythmias, and thromboembolic events, with clinical symptoms such as shortness of breath, fatigue, and fluid retention [3].
Problems involving the lymphatic system are particularly troublesome. Elevated venous pressure and lymphatic overload can lead to protein-losing enteropathy (PLE), causing hypoproteinaemia, oedema, and immunodeficiency. Plastic bronchitis, though less common, is a severe complication characterised by the formation of large airway casts, resulting in respiratory distress. Additionally, chylous effusions, such as chylothorax or chylous ascites, can cause respiratory and abdominal discomfort [4]. Lymphatic abnormalities, including lymphangiectasia and lymphatic obstruction, contribute significantly to these issues. Novel surgical techniques are being explored to address these complications. The Hraska procedure aims to alleviate the haemodynamic inefficiencies of the Fontan circulation and decompress the lymphatic system, offering a potential solution for challenging symptoms [5, 6].
We present a case of a patient with hypoplastic left heart syndrome (HLHS) who underwent the Norwood, Glenn, and Fontan procedures. After the staged treatment, he developed failing Fontan physiology and plastic bronchitis, prompting a multidisciplinary approach to manage his complex condition. The patient underwent the Hraska procedure (innominate vein turn down). Although symptoms of plastic bronchitis were mitigated, he required evaluation for heart transplantation due to single ventricle dysfunction.
A newborn boy, weighing 3.2 kg, was prenatally diagnosed with HLHS and underwent the modified Norwood-Sano procedure at 10 days of age. Postoperatively, he developed pharmacologically managed congestive heart failure and temporarily drained pericardial effusions. The interstage period was uneventful. He subsequently underwent the hemi-Fontan procedure and developed a right pleural effusion that was drained in the early postoperative period. At 2.5 years of age, he underwent cardiac catheterisation before Fontan completion. The haemodynamic assessment revealed a right ventricular end-diastolic pressure of 7 mm Hg and a mean pulmonary artery pressure of 12 mm Hg, with an oxygen saturation of 62% in the pulmonary artery. The pulmonary-to-systemic blood flow ratio (Qp : Qs) was 0.61, and pulmonary vascular resistance (PVR) was 2.1 Wood units (PVR index: 1.3 Wood units·m²). A Fontan completion using an intra-atrial lateral tunnel with a 4-mm fenestration was carried out. In the postoperative period, he developed short-lasting pleural effusions, which were drained effectively.
Starting 2 years after Fontan surgery, he developed progressive cyanosis, reduced exercise tolerance, and began coughing up bronchial casts characteristic of plastic bronchitis. Echocardiography revealed a dilated inferior vena cava (IVC) without typical respiratory collapse, dilated hepatic veins, a non-restrictive interatrial connection, and a Fontan tunnel dilated to about 2.5 cm with retrograde flow during the expiratory phase. Right ventricular inflow appeared normal, with trace tricuspid regurgitation (tricuspid annular plane systolic excursion [TAPSE] 8.14 mm). The left middle pulmonary artery measured 8 mm, and the right middle pulmonary artery measured 12 mm. Cardiac output was approximately 5.7 l/min/m². The previously created fenestration was absent.
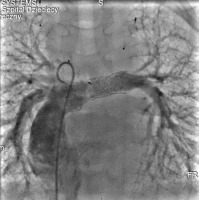
Cardiac catheterisation revealed a significant collateral vessel from the left inferior thyroid artery supplying the left lung and additional collateral vessels arising from the left (LIMA) and right (RIMA) internal mammary arteries supplying both lungs (Figure 1). The Fontan tunnel measured 14.1 mm at its junction with the IVC, widening centrally to 26 mm, and tapering to 10.9 mm at the junction with the pulmonary arteries. No fenestration was identified. The stent in the left pulmonary artery measured 9.8 mm proximally, increasing to 11.2 mm distally (Figure 2). During the procedure, an Amplatzer Vascular Plug successfully occluded the collateral vessel from the left inferior thyroid artery to the left lung, and vascular coils were placed in the RIMA and LIMA. Additionally, a 6-mm Valeo stent was implanted to create a new fenestration (Figure 3).
Figure 1
Angiogram of the left brachio-cephalic vein. The plug is inserted into the left mammary artery. Wide left brachio-cephalic artery (innominate artery), and the collateral vessel to the left lung (asterisk) are visible. A stent was placed in the left pulmonary artery
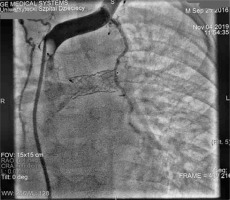
Figure 2
Angiography of the pulmonary arteries and Fontan tunnel. The tunnel measured 14.1 mm at its junction with the inferior cava vein, widened centrally to 26 mm and tapered to 10.9 mm at the junction with the pulmonary arteries. No fenestration was identified. The stent in the left pulmonary artery measured 9.8 mm proximally, increasing to 11.2 mm distally
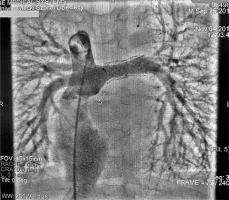
Figure 3
Angiogram of the pulmonary arteries and Fontan tunnel. The stent in the pulmonary artery was re-dilated, and a 6-mm Valeo stent was implanted to create a new fenestration
Haemodynamic measurements showed elevated pressures in the pulmonary circuit, which were suboptimal for Fontan circulation. The mean pulmonary artery pressure was 16 mm Hg, with a corresponding oxygen saturation of 53.2%. The right ventricular end-diastolic pressure was recorded at 9 mm Hg, pulmonary capillary wedge pressure (PCWP) at 10 mm Hg, and aortic pressures at 68/43 mm Hg, with an oxygen saturation of 94.7%. The Qp:Qs ratio was 0.93, indicating nearly balanced circulations. PVR was elevated to 4.2 Wood units (PVR index 2.5 Wood units·m²), and systemic vascular resistance (SVR) was significantly elevated to 24 Wood units. Despite these challenges, the catheterisation with fenestration reconstruction and collateral occlusion relieved the symptoms of plastic bronchitis. Therapy was initiated with phosphodiesterase-5 (PDE5) inhibitor and endothelin receptor antagonist, alongside anticoagulation, ACE inhibitor, and mineralocorticoid receptor antagonists.
Unfortunately, after 1.5 years, the symptoms recurred with worsening cyanosis, decreased exercise tolerance, and frequent infections with bronchial casts and productive cough. Repeated echocardiography revealed dilated IVC and hepatic veins, with a wide, unrestricted interatrial connection and a patent fenestration with a stent in place. The Fontan tunnel appeared wide. Following anterior leaflet prolapse, mild to moderate tricuspid regurgitation (grade I/II) was noted. TAPSE measured 9.6–9.7 mm, indicating mildly reduced right ventricular systolic function. The right ventricular free wall was thickened, but overall global and segmental function was good. There was trace aortic valve regurgitation, with the neo-aorta measuring approximately 20 mm in diameter. The superior cava and brachiocephalic veins were widened, and a broad connection was observed between the superior systemic veins and the pulmonary arteries. The stent in the left pulmonary artery (LPA) measured 8–8.5 mm in diameter, while the right pulmonary artery (RPA) measured 8–8.2 mm. The neo-aortic arch, including the isthmus, had a peak velocity ranging from 1.7 to 1.9 m/s. In the ultrasound of the abdomen, an enlarged liver with a fibrotic-like structure was depicted, indicating fibrotic remodelling of the liver.
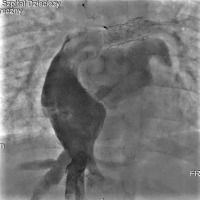
The subsequent heart catheterisation revealed a typical aorta without significant collateral vessels to the lungs. The innominate vein measured 8 mm in diameter, with no notable veno-venous anastomoses. The RPA measured 8.1 mm, while the LPA was approximately 8.5 mm in diameter and had undergone stent re-dilatation to 10 mm. The Fontan tunnel was dilated to about 3 cm, and the fenestration with a stent showed right-to-left shunting (Figure 4). Haemodynamic data showed a superior vena cava pressure of 20 mm Hg (oxygen saturation of 68%) and an IVC pressure of 22 mm Hg (oxygen saturation of 57%). The mean Fontan tunnel pressure was 20 mm Hg, and right ventricular pressures were 75/15 mm Hg. The RPA pressure was 20 mm Hg (oxygen saturation of 65%), while the LPA pressure was 18 mm Hg (oxygen saturation of 68%). The mean left atrial pressure was 16 mm Hg, with an oxygen saturation of 95%. The aortic pressure was 85/52 mm Hg, with an oxygen saturation of 85%. The calculated Qp : Qs ratio was 0.79, indicating reduced pulmonary flow. PVR was 3.8 Wood units (PVR index 2.28 Wood units·m²), and SVR was 38.13 Wood units, indicating significant haemodynamic strain typical of failed Fontan physiology.
Figure 4
Angiography of the pulmonary arteries and Fontan tunnel. The right pulmonary artery measured 8.1 mm and was dilated with the 12 mm balloon, and the left pulmonary artery had undergone stent re-dilatation to 10 mm. The Fontan tunnel was dilated to 3 cm in the central portion, and the fenestration with a stent showed right-to-left shunting. The hepatic veins and inferior vena cava were also significantly widened
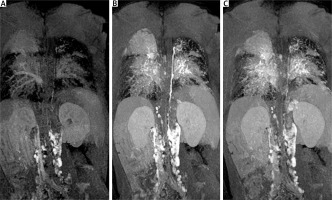
The 6-minute walk test showed reduced exercise tolerance, with a distance of 351 m and oxygen desaturation from 86% to 75%. Dynamic contrast magnetic resonance lymphangiography (DCMRL) revealed early opacification of the mesenteric lymphatic system, suggesting retrograde flow, with the absence of the cisterna chyli and cranial part of the thoracic duct. A tangled network of lymphatic structures was seen around the bronchi and trachea, indicating lymphatic abnormalities and high systemic venous pressure from the failing Fontan circulation (Figure 5).
Figure 5
Dynamic contrast magnetic resonance lymphangiography (DCMRL). A – Early opacification of the mesenteric lymphatic system, suggesting retrograde flow; B – delayed appearance of the thoracic duct and tangled network of lymphatic structures around the bronchi and trachea; C – late phase with remaining contrast indicating lymphatic abnormalities and high systemic venous pressure from the failing Fontan circulation
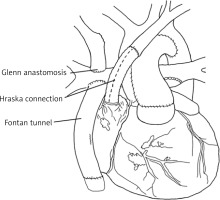
At 5 years of age, given the haemodynamic insufficiency of the Fontan circulation, a multidisciplinary heart team opted for the Hraska procedure with Fontan tunnel revision and tricuspid valve plasty. The procedure was carried out with cardiopulmonary bypass, hypothermia, and cold blood del Nido cardioplegia. The stent was excised from the fenestration, and the Gore-Tex tunnel was removed. The prolapse of the anterior leaflet of the tricuspid valve was corrected with effective valve plasty. A new Gore-Tex tunnel was implanted to direct flow from the IVC to the pulmonary arteries with a 4-mm fenestration. The left brachiocephalic vein was detached from the superior vena cava and anastomosed to the right atrial appendage using a 10-mm Gore-Tex graft to decompress the left jugular angle and thoracic duct into a low-pressure system (Hraska procedure, Figure 6).
Figure 6
Schematic picture of the Hraska procedure. The left brachiocephalic vein was severed from the superior vena cava and was anastomosed to the right atrial appendage using a Gore-Tex crimped vessel. Drawing based on Ann Thorac Surg 2013; 96: 709–711
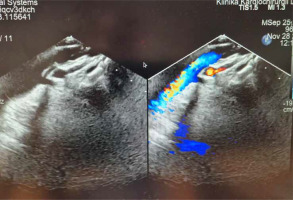
The patient recovered uneventfully, with oxygen saturation improving to 90% in the early postoperative period. He was discharged home on the 10th postoperative day with patent Hraska anastomosis (Figure 7) and mild systolic and diastolic right ventricular dysfunction on pharmacologic treatment.
Figure 7
Echocardiographic picture of patent Hraska anastomosis between the left brachio-cephalic (innominate) vein and right atrial appendage. The colour Doppler flow through the 8 mm reinforced polytetrafluoroethylene (Gore-Tex) vessel anastomosis is visible
The surgical intervention relieved the symptoms of plastic bronchitis, although the child continued to experience dyspnoea and audible stridor, especially after physical exertion. A subsequent bronchoscopy revealed malacia of the left main bronchus with a 50% narrowing and mucosal congestion. Despite ongoing medical management and close monitoring, his condition continued to deteriorate. Given the progression of symptoms of heart failure and the limited effectiveness of previous interventions, the patient was evaluated and found eligible for heart transplantation. He was transferred to a transplantation centre for further evaluation and management.
This case underscores the multifaceted challenges in managing HLHS after staged treatment ending with failing Fontan physiology, especially when complicated by severe lymphatic dysfunction manifesting as plastic bronchitis. The patient underwent the first Hraska procedure in Poland. Its clinical course illustrates the limitations of currently available interventions and the necessity for individualised multidisciplinary management strategies.
While life-saving for patients with single-ventricle physiology, the Fontan procedure establishes a non-physiological circulation characterised by elevated central venous pressure (CVP) [7]. The CVP was markedly elevated in our patient, with superior and inferior vena cava pressures recorded at 20 mm Hg and 22 mm Hg, respectively. This systemic venous hypertension leads to lymphatic hypertension and impaired drainage, which are fundamental in the development of complications such as plastic bronchitis and protein-losing enteropathy (PLE) [8].
Under normal conditions, the lymphatic system returns approximately 2 to 3 litres of protein-rich fluid from the interstitial spaces back to the bloodstream daily. The thoracic duct (TD) plays a critical role in this process, draining about 85% of the body’s lymph [9]. The increased lymph production seen in our patient overwhelmed the TD’s transport capacity, leading to lymphatic congestion and leakage into the airways. This resulted in lymphatic fluid entering the bronchial tree and forming obstructive casts.
Advanced imaging modalities were pivotal in assessing our patient’s condition. Dynamic contrast-enhanced magnetic resonance lymphangiography (DCMRL) revealed significant lymphatic abnormalities due to high systemic venous pressure from the failing Fontan circulation. Recent studies highlight that imaging techniques are essential for visualising lymphatic architecture and guiding interventional and surgical planning [10, 11].
Initial management included catheter-based interventions to address haemodynamic inefficiencies. Occlusion of collateral vessels supplying the lungs aimed to reduce unnecessary pulmonary blood flow, while the re-creation of a fenestration sought to decompress the Fontan circuit. Pharmacological therapy was initiated to reduce pulmonary vascular resistance. Despite temporary symptomatic relief, these measures were insufficient to halt the progressive decline in our patient’s condition.
Given the persistent lymphatic complications, the multidisciplinary team opted for the Hraska procedure. This surgical intervention aims to decompress the lymphatic system by redirecting the left brachiocephalic (innominate) vein to the right atrium, thereby reducing outflow pressures in the TD [12]. In our patient, this involved detaching the left brachiocephalic vein from the superior vena cava and anastomosing it to the right atrial appendage using a Gore-Tex graft. Postoperatively, the patient experienced relief from plastic bronchitis symptoms and an improvement in oxygen saturation, which was consistent with the reported benefits of TD decompression. However, the patient’s symptomatic improvement was short-lived; the persistent ventricular dysfunction and progression of symptoms underscored the limitations of the procedure in addressing underlying ventricular dysfunction or poor myocardial contractility.
Studies have shown that the Hraska procedure may be more effective in patients whose lymphatic complications are primarily due to elevated CVP rather than intrinsic ventricular dysfunction. In cases like ours, where ventricular failure is a significant contributor, the procedure may provide only temporary relief [13]. Given the progression of our patient’s condition despite maximal medical and surgical interventions, heart transplantation was deemed necessary. This decision aligns with the current understanding that heart transplantation remains the definitive treatment for patients with failing Fontan physiology who do not respond to other therapies. Early identification and timely referral for transplantation are crucial, as delays can lead to worsening end-organ function and adverse outcomes.
In conclusion, the Hraska procedure provides a valuable surgical option for alleviating symptoms associated with failing Fontan physiology, particularly lymphatic complications such as plastic bronchitis. While it can significantly improve quality of life and offer symptomatic relief even during the wait for heart transplantation, it does not treat underlying heart dysfunction or poor ventricular contractility. Recognising its limitations and ensuring timely referral for heart transplantation are essential for patients whose conditions continue to deteriorate despite such interventions.






