Introduction
Aortic valve stenosis (AVS) accounts for approximately 6% of all congenital heart defects, with a significant (3–5-fold) predominance in males [1]. Its rarest form, isolated, critical neonatal AVS, is a cardiac emergency. The choice of first line treatment for these newborns – transcatheter intervention or cardiac surgery – is still debatable, although at this age both methods are palliative [2].
Aim
The aim of the study was to assess the immediate and long-term outcome of balloon aortic valvuloplasty (BAV) procedure in the treatment of critical AVS in newborns including preterm infants.
Material and methods
Data were collected on all patients hospitalized in the years 2004–2015 in the Department of Pediatric Cardiology, University Hospital in Krakow. The study was based on a retrospective registry of patients diagnosed and treated according to local standards, so ethics committee approval was not required.
The inclusion criteria were the following: newborns, congenital heart defect: isolated critical aortic valve stenosis (with or without patent ductus arteriosus), balanced size of both ventricles, percutaneous balloon aortic valvuloplasty as an initial therapy before 1st month of life, follow-up > 5 years.
The exclusion criteria included: congenital lethal malformations, complex congenital heart defect with critical aortic stenosis and coexisting heart defect (with or without patent ductus arteriosus), unbalanced size of both ventricles, surgical valvuloplasty as an initial therapy, follow-up < 5 years.
BAV was performed under general anesthesia. In most patients (26 of 28), vascular access was obtained through the femoral (mainly right) artery, while in 2 patients it was through the left common carotid artery, then through the ascending aorta and retrogradely to the valve. We used Tyshak II balloon catheters (NuMED Inc. USA) with a diameter of 4–7 mm. The size of the balloon was selected depending on the diameter of the aortic annulus measured angiographically (balloon/annulus ratio ≤ 1.0).
Statistical analysis
Categorical variables are presented as numbers and percentages. Continuous variables were expressed as mean, standard deviation (SD) or median and first (25%) and third (75%) quartile (Q1-Q3). Differences between groups were compared using the non-parametric Wilcoxon test. A comparison of the change of selected parameters over time was performed using a paired t-test or a Wilcoxon signed rank test.
Kaplan-Meier curves for selected groups and parameters were compared using log-rank and Wilcoxon tests. Categorical variables were compared by Pearson’s c2 test. The risk factors for the mortality were analyzed based on nominal logistic regression. Two-sided p-values < 0.05 were considered statistically significant. All calculations were done with JMP, Version 16.1.0 (SAS Institute).
Results
A total of 29 symptomatic neonates with critical AVS were treated in our center between January 2004 and December 2015. Surgical treatment was performed in one newborn (with well-defined AV commissures), while the remaining 28 patients (19 M + 9 F), including 7 premature infants, underwent a BAV procedure.
The patients were divided into two groups depending on the left ventricle ejection fraction (LVEF) value: group I with LVEF ≤ 40% (12 patients) and group II with LVEF > 40% (16 patients). Morphological features of the bicuspid AV valve were found in 22 neonates, including 8 (66.7%) from group I and 14 (87.5%) from group II. Another 4 newborns had a 3-leaflet valve, including 3 (25%) in group I and 1 (6.3%) in group II. In the remaining 2 neonates (1 from each group), its type was not determined. Basic data of the studied patients in group I and group II are shown in Tables I and II. There were 5 premature infants in group I and 2 in group II. The initial value of transvalvular pressure gradient across the aortic valve (TAPG) in the Doppler study in neonates from group I was 56.1 mm Hg and from group II 80.1 mm Hg, while during catheterization it was 56.3 mm Hg and 60.0 mm Hg, respectively.
Table I
Baseline characteristics of study population
Table II
Characteristics of patients at time of BAV procedure
The results of BAV procedures in these groups are presented in Table III. All children were treated for congestive heart failure. Additionally, 9 (32.1%) newborns were treated with Prostin VR, and 1 required vasopressor and inotropic support.
Table III
Effect of BAV treatment in study groups
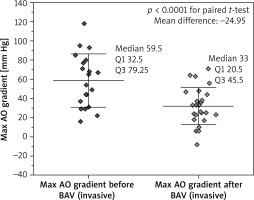
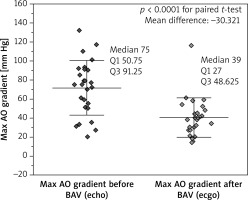
The value of TAPG after BAV decreased significantly in both groups. Comparison of the results in invasive and echocardiographic studies is shown in Figures 1 and 2, respectively. In the invasive examination the mean difference in TAPG before and after BAV in patients from group I was – 22.1 mm Hg and in patients from group II – 26.5 mm Hg, while in the Doppler exam these values were 22.2 mm Hg and 36.1 mm Hg, respectively. A pressure gradient < 35 mm Hg was present in 8 (66.7%) patients in group I and in 7 (43.8%) patients in group II (p = 0.0956, Pearson’s c2 test). In echocardiographic studies, the mean LVEF value after BAV in group I patients increased significantly (the mean difference was 11.2%), while in group II patients this difference was non-significant.
Various intraprocedural complications occurred in 6 (21.4%) patients, including 1 patient from group I and 5 patients from group II. We detected LV wall perforation (requiring pericardial drainage) in 2 patients, mild AV perforation in 1 patient (from group I), prolonged post-procedural bleeding requiring blood transfusion in another 2 patients, and clinically significant (third degree) aortic regurgitation (AR)in 1 patient. Post-procedural moderate (second degree) AR occurred in 9 newborns (2 from group I and 7 from group II). The patients were followed up a day after the procedure, during their hospital stay as needed, and after a discharge from hospital, every 3 to 6 months, depending on any residual findings.
Twelve (42.8%) children (5 from group I including 3 of 5 preterm infants and 7 from group II including 1 of 2 premature infants) required a total of 14 various re-interventions, performed 0.2–123 months after initial BAV (mean: 30.3 months, median: 16.5 months). The following procedures were performed: repeated BAV in 4 children (including 1 child from the premature subgroup who underwent AV replacement, and finally Ross-Konno operation) and surgical valvuloplasty in 8 patients.
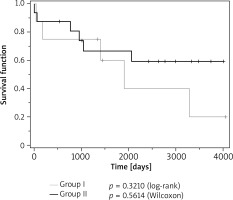
Re-interventions were comparably frequent in patients from both groups (group I: 41.7% vs. group II: 43.7%), but more frequent in children born prematurely than in children born at term (57.1% vs. 38%, respectively) (Figure 3).
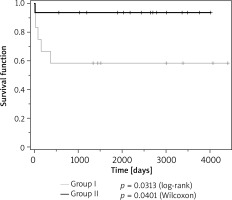
A total of 6 patients died (5 infants from group I and 1 from group II (p = 0.0238, Pearson’s c2 test) aged 3 weeks to 1 year, mean = 3.8 months, median = 2 months. Survival in the study groups is shown in Kaplan-Meier plots in Figure 4.
There were 3 deaths out of 7 (42.8%) children from the preterm group and 3 out of 21 (14.3%) children from the full-term group (p = 0.1106, Pearson’s c2 test). The main cause of death was infective complications superimposed on refractory congestive heart failure. It was found that baseline systolic LV dysfunction, defined as LVEF ≤ 40%, increases the risk of death ten-fold (OR = 10.7, 95% CI: 1.1–109.8; p = 0.0458) compared to LVEF > 40%.
The average follow-up period was 6.6 years (SD: 3.2 months; median: 7.2 years, Q1–Q3: 4–8.5). The clinical condition of all 22 patients receiving outpatient care was good, classified as NYHA class I or II. One of them, a 12-year-old boy with progressive AR, was found eligible for surgical valvuloplasty. The mean LVEF value at the end of the follow-up period was 69.0 % (SD: 8.8%, median: 72%, Q1–Q3: 62.3–76%). The difference between the mean LVEF value (56.7%) assessed in patients immediately after BAV and the mean LVEF value (69%) assessed in patients at the end of follow-up was significant (12.3%, p = 0.0363 for paired, two-sided t-test).
Discussion
Isolated critical AVS in newborns is the rarest form of this heart defect, constitutes an emergency and requires urgent treatment. Over a 11-year period (2004–2015), a total of 29 newborns with this heart defect were treated in our center. An almost identical annual incidence of this defect (103 newborns in the years 1989–2015) was found in the study by Vergnat et al. [3]. Over half of their patients (52) were treated with open valvulotomy (OV), while the remaining 51 underwent BAV. They concluded that OV may provide a better individualized approach than “blunt BAV” and better serve long-term outcomes. In our study, only one newborn had well-defined aortic valve (AV) commissures, favoring heart surgery. In 22 (78.6%) of our patients, the AV had bicuspid morphology (67% of group I patients and 87.5% of group II patients). In 4 patients (14.3%, including 3 neonates from group I and in 1 from group II) the AV was deformed, morphologically trileaflet. In the remaining 2 (7.1%) patients it was not specified. Many pediatric heart centers currently favor BAV for the first-line treatment of such defect in newborns. The reasons are rapid relief of stenosis, avoidance of sternotomy and other surgery-related traumas, and faster recovery. The most common complication of the BAV procedure is AR, but a severe degree of this event is rarely seen [4]. This complication occurred in only 1 (3.6%) of our patients. In a newborn, whose valve was accidentally perforated by the guidewire during the procedure, the resulting AR was mild (first degree). Moderate AR (second degree) following BAV occurred in 9 of 28 (32.1%) newborns. During an average follow-up period of 6 years, an increase in AR was observed in only 1 patient. However, it is likely to increase in the long run. In addition to significant AR in 1 newborn and accidental AV perforation in another one, other adverse events occurred in 4 further patients. These included LV perforation in 2 patients and prolonged bleeding from the catheter insertion site in the remaining 2 patients. Adverse events were more common in group II patients (1 from group I and 5 from group II). This is difficult to explain and is probably coincidental, as in most cases (4 of 7) they were not related to AV itself. A total of 12 (42.8%) children required re-intervention at a mean of 30.3 months after initial BAV. The rate of re-intervention was slightly higher in the preterm subgroup (57.1%) than in the full-term subgroup (38%). This difference remains unclear, because the principles of performing the BAV procedure in all newborns were identical. Moreover, the number of patients undergoing re-treatment in the study groups was too small to make a statistical assessment. Tyc et al. reported a lower (35.5%) rate of re-interventions, but their material was more homogeneous [5]. In the literature there was no statistically significant difference between BAV and surgical valvotomy in terms of the need for re-intervention [6].
Bonello et al., in their 40-year (1970–2010) retrospective review of 96 consecutive patients who required neonatal (< 30 days) intervention for AVS, revealed that early deaths occurred in 19 (19.8%) patients, and freedom from re-intervention at 10 years was 41.8% [7].
The limitation of BAV is inaccuracy of the expanding balloon impact on the stenotic valve, increasing the risk of residual changes and/or adverse events, with the necessity of earlier re-intervention [8, 9]. Apart from isolated cases, there are no collective data on the outcomes of BAV in premature newborns with severe AVS. In our study, 7 of 28 (25%) newborns were premature. In general, prematurity itself favored the BAV procedure rather than surgery. On the other hand, the impact of the expanding balloon on the stenotic valve is not entirely predictable and may be ineffective, resulting in residual AV stenosis, or excessive, leading to its significant regurgitation [10, 11]. Additionally, OV allows for precise separation of leaflets and correction of their deformations, which BAV does not offer [12–14]. Either way, critical AVS is a lifelong defect, and surgical or interventional treatment for this form of AVS in newborns is a palliative, life-saving procedure. We were able to compare our results of BAV in newborns to the results of this method in other pediatric heart centers due to the small number of operations of this defect in our center. Many series denote that balloon/annulus ratio ≤ 1.0 is the best for reduction of significant AR. There are some other independent predictors for the significant post-BAV aortic regurgitation such as larger valve size, dysplastic changes of smaller valves, and preexisting, even mild AR [15].
Poor outcome of BAV included ductal-dependent blood flow, concomitant mitral valve stenosis, borderline LV (which did not form the apex of the heart), extensive endocardial fibroelastosis and aortic valve diameter < 6 mm. Neonatal mortality was due to procedural difficulties, myocardial contractility impairment and infectious complications. The highest risk patients are the neonates with significant LV systolic dysfunction, which is confirmed by our experience. The most common serious procedural complication is LV wall perforation with a guidewire [13, 14]. The smaller the child, the higher is the likelihood of vascular complications. Carotid and umbilical artery access in newborns may decrease the risk of vascular events. Zain et al. reported a neonatal series in which 33% had femoral artery thrombosis [13]. There were no such events in our material. Small balloons (with mean balloon/annulus ratio 0.9) did not seem to prevent against significant AR.
There is no generally accepted definition of critical AVS in newborns, distinguished mainly by PDA-dependent systemic flow requiring PGE1 infusion and/or adjunctive inotropic/catecholamine therapy. Our experience to date with severe AVS in newborns shows that, regardless of the initial symptoms, they are at high risk of rapidly developing serious heart failure. Therefore, any case of severe AVS at this age should be considered a “critical defect” requiring immediate treatment.
The present work has several limitations. The most important are its retrospective character and relatively small number of patients, due to the generally rare occurrence of critical AVS in newborns. Moreover, the lack of standardization of echocardiography assessment and invasive hemodynamic measurements may introduce differences in assessments between individual operators, but it reflects well the results of everyday work.
Conclusions
Critical AV stenosis in neonates requiring urgent intervention is relatively rare. BAV as the initial treatment of this defect gives good immediate results, comparable to surgical ones. However, further observations have shown the need for frequent re-interventions, especially in patients born prematurely. Mild to moderate AR after the procedure seems to be insignificant in the medium- to long-term follow-up. Severe baseline reduced LVEF, and prematurity are risk factors for higher mortality, but the latter increases it insignificantly.