Summary
Data linking anemia in patients undergoing percutaneous coronary intervention (PCI) and adverse outcomes in a long-term follow-up are limited. In our study, the incidence of anemia in patients with multivessel coronary artery disease undergoing PCI was relatively high and co-existence of anemia was associated with poorer outcomes in 6-year follow-up. An important risk factor for stratifying PCI patients was revealed and the importance of assessing hemoglobin levels was underlined.
Introduction
Coronary artery disease (CAD) remains one of the major problems of contemporary medicine, with a considerable impact on morbidity, mortality and the healthcare system. Despite significant advances in its treatment, CAD remains the leading cause of death among middle-aged and elderly individuals. It is estimated that in 2020 CAD affected 244.1 million people worldwide and was the leading cause of cardiovascular (CV) mortality, responsible for 9.44 million deaths in 2021. In our center, a real-life study investigating outcomes of patients with severe CAD after implementing various treatment strategies was performed [1–4].
In addition to the well-known risk factors, hematological disorders also seem to play an important role in the progression and severity of CAD. Anemia is one of the most common hematological abnormalities observed in patients with CAD. The most common causes of anemia in elderly individuals are chronic diseases (35%), iron deficiency (15%), blood loss (7%), renal failure, liver disease and endocrine disease (6.5%), myelodysplasia, leukemia (5.5%), and vitamin B12 and folic acid deficiency (5.5%) [5]. Anemia occurs in 24.6% of patients undergoing primary percutaneous coronary intervention (PCI) [6, 7]. The progressive aging of the population is leading to an increase in the number of patients with CAD and anemia [8]. Furthermore, patients with CAD undergoing PCI are susceptible to an imbalance between oxygen supply and demand during the procedure, which will be exaggerated in the presence of anemia. The outcomes of PCIs are largely dependent on various factors, including age, coronary lesion complexity, the extent of peripheral atherosclerosis, the severity of heart failure (HF), chronic kidney disease (CKD), diabetes or the presence of left main disease (LMD) or three-vessel disease (3-VD).
Aim
The aim of the present study was to assess the impact of anemia on clinical outcomes in patients with multivessel CAD treated with percutaneous coronary intervention in long-term follow-up.
Material and methods
In this retrospective study we examined 6-year outcomes: primary and secondary endpoints of 679 individuals with multivessel CAD treated with PCI based on the hemoglobin (HGB) value before the interventional procedure.
This single-center observational study was conducted in the 1st Department of Cardiology, Medical University of Warsaw, a large tertiary cardiovascular care center in Poland. The inclusion criteria were age ≥ 18 years and complete clinical, echocardiographic and angiographic characteristics. The exclusion criteria included the following: pregnancy/lactation, disseminated neoplastic process, life expectancy < 1 year and lack of informed, written consent.
Management of patients was conducted according to current recommendations. All experiments and analyses were performed in accordance with the relevant guidelines and regulations.
3-VD was defined as stenosis greater than 70% or between 40 and 70% but assessed with functional tests (fractional flow reserve (FFR), instantaneous wave-free ratio (iwFR) or quantitative flow ratio (QFR)) as hemodynamically significant in at least three vessels with 1.5 mm or more in diameter, while left main (LM) disease was defined as LM stenosis of 50% or higher. HGB level was based on blood draws before the procedure. Mortality data were obtained from review of medical records or follow-up phone call to the family. Angiographic data were collected from all patients undergoing PCI and recorded in the Cardiovascular Information Registry. The SYNTAX score was calculated for all patients without prior coronary artery bypass grafting (CABG).
The primary endpoint was overall mortality, while major adverse cardiac or cerebrovascular events (MACCE) (i.e. overall mortality, stroke, myocardial infarction (MI), or repeat revascularization (RR)) and separate components of MACCE were defined as secondary endpoints. Major bleeding was defined as 3 or more on the BARC (Bleeding Academic Research Consortium) scale.
The World Health Organization defines anemia as a HGB level lower than 13 mg/dl for men and 12 mg/dl for women [9]. In this analysis, we defined anemia as a HGB level lower than 13.8 mg/dl for men and 12 mg/dl for women according to the standards adopted by our central laboratory.
Statistical analysis
Statistical analysis was performed using the PQStat software (version 1.6.6, PQStat, Poznan, Poland).
The normality of distribution for continuous variables was confirmed with the Shapiro-Wilk test. Categorical data were expressed as counts and percentages, while continuous data were presented as means with standard deviation (SD). Group comparisons were performed using the χ2 test for categorical variables, Student’s t test or the Mann-Whitney U test for unpaired continuous variables, and the Wilcoxon rank-sum test for paired variables, depending on data distribution. Hazard ratios (HRs) with 95% confidence intervals (95% CI) were calculated to compare outcomes across all strategies. Time-to-event analysis was conducted using Kaplan-Meier curves. All p-values were reported as 2-sided, and a p-value of 0.05 or lower was considered statistically significant.
Results
Study population
Baseline clinical characteristics (overall and by groups) in detail are summarized in Table I.
Table I
Baseline clinical characteristics
[i] PCI – percutaneous coronary intervention, BMI – body mass index, ACS – acute coronary syndrome, LVEF – left ventricle ejection fraction, CCS – Canadian Cardiovascular Society, NYHA – New York Heart Association, TIA – transient ischemic attack, MI – myocardial infarction, PAD – peripheral artery disease, CKD – chronic kidney disease, COPD – chronic obstructive pulmonary disease, PH – pulmonary hypertension, EuroSCORE II – European System for Cardiac Operative Risk Evaluation II, STEMI – ST-segment elevation myocardial infarction, STS – Society of Thoracic Surgeons score.
The median (Q1, Q3) age of the overall cohort was 70 (63,76) years; 72.9% were men. Regarding periprocedural risk, the median (Q1, Q3) values of EuroSCORE II (European System for Cardiac Operative Risk Evaluation II) and the STS (Society of Thoracic Surgeons) score were 5.8% (2.4, 8.1) and 3.6 % (1.8, 5.3), respectively. Approximately 32.3% of patients had medically treated diabetes and 11.2% required insulin. The prevalence of hypertension, dyslipidemia and heart failure was relatively high in the overall cohort – 84.8%, 81.9% and 73.3%, respectively. We classified the participants into two groups: anemia and non-anemia. We found that 35.4% (240 out of 679) of the patients undergoing PCI were anemic. Patients with anemia were statistically significant older (median years (Q1, Q3) = 78 (75, 80) vs. 66 (59, 70) for non-anemia, p < 0.001). The incidence of impaired renal function was significantly higher in anemic than non-anemic participants (67.5% vs. 9.6% for non-anemia, p < 0.001). Patients with anemia showed a statistically significantly higher percentage of female gender (53/240 (22.1%) vs. 131/439 (19.8%) for non-anemia, p = 0.03) and frailty (35% vs. 1.1%, p < 0.001). There were no significant differences in rates of diabetes, hypertension and dyslipidemia between the two groups. Patients with anemia were more often current smokers (69/240 (28.8%) vs. 63/439 (14.4%), p < 0.001) and had chronic obstructive pulmonary disease (COPD) (70/240 (10.3%) vs. 22/439 (5%), p < 0.001). 11.3% of participants had hemodynamically significant stenosis of carotid arteries, while 8.8% had experienced a previous stroke. Regarding statistically significant differences in co-morbidities between the two groups, patients with anemia were generally more burdened and more often had severe pulmonary hypertension (PH) (10.8% vs. 4.8%, p < 0.001), chronic kidney disease (CKD) (67.5% vs. 9.6%, p < 0.001), atrial fibrillation (AF) (44.2% vs. 18.9%, p < 0.01), peripheral artery disease (17.9% vs. 1.4%, p < 0.001)) and active cancer (10.8% vs. 0.2%, p < 0.001). Furthermore, 42% of participants presented with ACS, 4.3% with cardiogenic shock, while the rest of them had chronic symptoms of CAD with more severe angina symptoms (Canadian Cardiovascular Society (CCS) class III–IV) in the anemia group (53.8% vs. 31.2%, p < 0.001). The rates of non-ST-segment elevation MI were higher in the anemia group (37.5% vs. 17.5% for non-anemia, p < 0.001). The history of previous MI was higher in patients with anemia (67.1% vs. 35.8%, p < 0.001), with a higher rate of prior revascularization in patients with anemia (57.5% vs. 34.2%, p < 0.001), both with a history of previous PCI (39.6% vs. 27.8%, p = 0.002) and CABG (17.9% vs. 6.4%, p < 0.001). The prevalence of HF was similar between the groups. Complete revascularization was achieved in 58.5% of patients, more frequently in the non-anemia group (62.2% vs. 51.7% for PCI; p = 0.008).
Regarding some variables of laboratory parameters related to morphology, etiology of anemia and iron deficiency, anemic patients as compared with the non-anemic cohort had significantly lower median (Q1, Q3) hemoglobin (g/dl), red blood cells (RBC, × 106/μl) and thrombocytes (×103/μl) levels (10.8 (9.8, 11.4) vs. 13.7 (13.1, 14.5), 3.7 (3.3, 4.1) vs. 4.8 (4.5, 5.1) and 135 (111, 161) vs. 188 (151, 232), respectively, p < 0.001); lower mean cell volume (MCV, fl), iron concentration (micrograms/deciliter) and transferrin saturation (TS, %) (81 (74, 86) vs. 89 (83, 92), 41 (27, 78) vs. 105 (76, 152) and 21 (19, 24) vs. 29 (25, 35), respectively, p < 0.001), while ferritin concentration (μg/l) was significantly higher for anemic-patients (338 (191, 376) vs. 168 (135, 197), respectively, p < 0.001). White blood cell (4–11 ×103/μl) level was comparable between groups (6.5 (4.1, 8.5) vs. 6.5 (5.8, 9.5) for non-anemic, respectively, p = 0.77).
Angiographic parameters
Overall, the median (Q1, Q3) number of affected lesions was 4 (2.9, 5.4), 23.3% of patients had significant LMD, 72.3% coronary stenosis involving bifurcation, 30.5% severe calcification and 24.2% at least one artery considered chronically occluded (CTO). The median (Q1, Q3) SYNTAX score for the overall cohort was 33 (22.9, 34.8) points. Patients with anemia showed a statistically significantly higher number of affected lesions (median (Q1, Q3) = 4.8 (3.8, 5.8) vs. 3.8 (2.9, 4.8) for non-anemia, p < 0.001). LMD was found more frequently in the anemia group (36.7% vs. 15.9% for non-anemia, p < 0.001). Patients with anemia also had more calcified arteries (45% vs. 22.6% for non-anemia, p < 0.001) and more often coronary stenosis involving bifurcation (84.6% vs. 65.6% for non-anemia, p < 0.001). The SYNTAX score was significantly higher in the anemia group (median years (Q1, Q3) = 33.8 (23.8, 34.8) vs. 23.8 (22.9, 33.8) for non-anemia, p < 0.001). The angiographic parameters are detailed in Table II.
Table II
Angiographic parameters
Outcomes
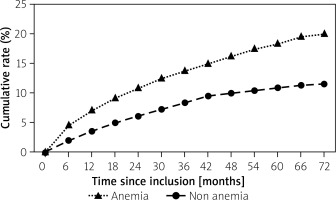
The ratios of all-cause death and MACCE in the overall cohort after 6 years were 99/679 (14.6%) and 388/679 (57.1%), respectively. The occurrence of the primary endpoint significantly differed between anemia and non-anemia-groups (48/240 (20.0%) vs. 51/439 (11.6%), p = 0.003) – Figure 1. The rate of cardiovascular (CV) mortality was comparable between the groups (29/240 (12.1%) vs. 48/439 (10.9%) for the non-anemia cohort, respectively, p = 0.65), while rates of non-CV death and bleeding death were higher for anemic patients (19/240 (7.9%) vs. 3/439 (0.7%) for the non-anemia cohort, p < 0.001 and 6/240 (2.5%) vs. 1/439 (0.2%), p = 0.005, respectively). The incidence of stroke was similar between groups (10/240 (4.2%) vs. 13/439 (3.0%) for the non-anemia cohort, p = 0.41). There was also no difference between anemia and non-anemia groups in terms of repeat revascularization (78/240 (32.5%) vs. 115/439 (26.2%), p = 0.28). The co-existence of anemia was associated with increased rates of MACCE, MI and in-hospital mortality (177/240 (73.8%) vs. 211/439 (48.1%); 51/240 (21.3%) vs. 44/439 (10.0%) and 21/240 (8.8%) vs. 4/439 (0.9%) for non-anemia, p < 0.001 for all, respectively). The incidence of major bleeding was significantly higher in the anemia cohort (22/240 (9.2%) vs. 9/439 (2.1%), p < 0.001). Also, postprocedural hospital stay was significantly longer in the anemia group (median (Q1, Q3) 6.6 (1.5) vs. 2.8 (1.0) days, respectively, p < 0.001). The primary and secondary endpoints are detailed in Table III.
Table III
Primary and secondary endpoints
Discussion
Our study found that 35.4% of the patients undergoing PCI were anemic. Many other studies have shown that the prevalence of anemia in patients undergoing PCI varies from 10% to 30% [10–14]. In our study anemic patients undergoing PCI were older, mostly female and more burdened in terms of co-morbidities including COPD, severe PH, AF and CKD. However, in our study there were no significant differences in rates of diabetes, hypertension and dyslipidemia between patients with and without anemia. A large study conducted on 10,658 Chinese patients undergoing PCI revealed that patients with anemia undergoing PCI had a significantly higher incidence of diabetes, renal impairment, old age, ACS and severe CAD. A higher incidence of female gender, old age and renal failure in anemic patients undergoing PCI was also found in a study conducted on 2819 patients [15, 16].
In a study that evaluated the association between baseline HGB concentration and a range of cardiovascular clinical outcomes in a cohort of nearly 40 000 patients enrolled in clinical trials across the spectrum of ACSs, anemia was a powerful predictor of cardiovascular mortality and ischemic events. The study revealed an independent relationship between HGB and CV end-points at 30 days [6]. In 629 patients with NSTEMI in intensive care units almost 1 in 3 patients had anemia at admission. Patients with anemia had a higher incidence of mortality or readmission at 6 months, and this association was independent from potential confounders [17]. A study conducted on 300 patients with newly diagnosed CAD revealed a decreased HGB level in 11.3% of participants and showed that HGB level was an independent predictor of MACCE in the investigated population [18]. Another study analyzed the relationship between HGB level and outcomes in 5010 patients with acute MI complicated by HF in the OPTIMAAL study. In 3921 patients, for whom follow-up HGB levels were available, mortality and hospitalization rates for patients admitted with MI complicated by HF and low HGB were notably higher over a 3-year follow-up period [19].
The main finding of the present study is that presence of anemia was associated with higher post-PCI mortality in 6-year follow-up. However, what also could be more important is the fact that the incidence of CV mortality was similar in both groups, while the rates of non-CV death and bleeding death were significantly higher for anemic patients. This phenomenon may be, at least in part, due to the fact of a significantly higher percentage of patients with active cancer in the group of anemic patients, but certainly also related to higher rates of major bleeding in this group. Data linking anemia in patients undergoing PCI and adverse outcomes in a long-term follow-up are limited. In another study of 6,929 consecutive patients treated with PCI, 24.6% had anemia according to criteria of the World Health Organization (WHO). Patients who had anemia compared with those who did not have anemia had significantly (p < 0.0001) higher mortality rates during hospitalization (1.9% vs. 0.4%) and at 1 year (12.8% vs. 3.5%) [6]. The CADILLAC trial enrolled 2,027 patients with AMI undergoing PCI to compare the adverse effects of anemia. Anemia was present in 260 (12.8%) randomized patients with baseline laboratory values. Anemia at admission was associated with higher mortality at hospitalization (4.6% vs. 1.1%, p = 0.0003), 30-day follow-up (5.8% vs. 1.5%, p < 0.0001) and 1-year follow-up (9.4% vs. 3.5%, p < 0.0001). The rates of disabling stroke at 30 days (0.8% vs. 0.1%, p = 0.005) and at 1 year (2.1% vs. 0.4%, p < 0.001) were also significantly higher in the presence of anemia. In multivariate analysis, anemia was an independent predictor of in-hospital mortality (HR = 3.26; p = 0.048) and 1-year mortality (HR = 2.38; p = 0.016) [13]. Based on a study conducted on 193 male patients presenting with ACS, baseline anemia was a strong and independent predictor of death or AMI at 2 years. At 24 months, the event-free survival was 64% in the group with a HGB level < 13 g/dl compared with 81% in the group with a HGB level > 13 g/dl [20]. In 6116 patients with either stable CAD or an MI, the presence of baseline anemia at the time of PCI was associated with higher 30-day major adverse cardiac events and longer length of stay, and, after controlling for multiple covariates, a significant difference in 1-year survival was noted in the anemic groups compared with the no-anemia group [12]. Another study evaluated the relationship between anemia and outcomes of PCI. Data on 48,851 consecutive PCIs were prospectively collected; 11,130 patients were anemic. Anemic men and women were older and had a higher percentage of comorbidities compared with their nonanemic cohorts (p < 0.0001 for all comparisons). After adjustment for comorbidities, anemia was associated with a higher risk of in-hospital mortality (odds ratio (OR) = 2.29; 95% CI: 1.79 to 2.92; p < 0.0001), MI (OR = 1.34; 95% CI: 1.05 to 1.72; p = 0.02) and major cardiovascular events (OR = 1.2; 95% CI: 1.05 to 1.34; p < 0.01) [21].
We also collected some data regarding laboratory parameters related to the morphology and etiology of anemia and iron deficiency in anemic and non-anemic cohorts (Table I). Taking into account all available variables, we concluded that the etiology of anemia in the anemic group was mostly related to the co-existence of many chronic diseases and the associated inflammatory process, and to a lesser extent with iron deficiency.
The mechanisms and pathophysiology of anemia in CAD are complex and multifactorial [22]. Regarding the relationship between anemia and CAD, the most common theory is that acute or chronic anemia can lead to systemic hypoxia and myocardial ischemia [23]. In anemia, hemodynamic changes include increased systemic arterial dilation, decreased systemic vascular resistance, reduced afterload, and eventually an increased stroke volume [24]. Nonhemodynamic changes include increased erythropoietin production [25]. Anemia elevates the risk of arrhythmias, MI, and myocardial fibrosis primarily by increasing cardiac output (which leads to an increase in LV mass and, in some cases, LV end-diastolic volume dilation). In some cases, severe anemia may participate in type 2 MI.
Interestingly, we noted that patients with anemia had greater anatomical progression of CAD. The potential explanation for this phenomenon could involve investigating the relationship between anemia and immune status. Some studies suggest that anemia of chronic diseases or related to iron deficiency is strongly associated with immune activation, which can lead to atherosclerosis progression and adverse outcomes in CAD patients. Anemic patients were found to have elevated levels of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), interferon γ (INF-γ), high-sensitivity C-reactive protein (hsCRP), interleukin 6 (IL-6), interleukin 12 (IL-12), fibrinogen, neopterin and serum amyloid A (SAA) – all of them enhance macrophage activation, accelerate the progression of atherosclerosis and thus are associated with severity of CAD [26]. This led to the conclusion that anemic patients with high concentrations of pro-inflammatory cytokines are exposed to more accelerated progression of atherosclerosis and have more advanced coronary artery disease (calcifications, percentage of left main disease, SYNTAX score).
According to current ESC guidelines, there is still no established strategy for treating anemia in patients with ACS. However, it is important to emphasize that anemia may be an important marker of sicker patients, thus making it difficult to fully adjust for all the potential confounding variables that may go unmeasured. Whether optimization of HGB before PCI is of clinical benefit will need to be determined in a randomized clinical trial.
Despite the advantages of this study, its limitations should be noted. Even though we collected and analyzed patients’ characteristics with reasonable accuracy, the risk of bias could be greater than within randomized controlled trials. The differences between baseline characteristics of study cohorts should be underlined, although these disparities were expected due to the nature of this research.
We did not further investigate the relationship between the severity of anemia and outcomes. Although a rigorous analysis was conducted to adjust for other confounders, we cannot entirely rule out the possibility that some unknown confounders were not accounted for and that therefore anemia is just an indirect marker of disease severity. Another limitation is the exclusion of medications taken by patients, including the type and duration of antiplatelet therapy, from our analysis.
Conclusions
Our study revealed an important risk factor for stratifying PCI patients. Further research is needed to confirm the role of low HGB levels and anemia in CAD patients undergoing PCI and to explore the potential impact of anemia treatment on patient prognosis. As the incidence of anemia in patients with CAD undergoing PCI is relatively high, HGB levels should be evaluated upon admission and considered in risk stratification.