Summary
We have analyzed the impact of the ovality index (OI) of pulmonary veins (PVs) on cryokinetic parameters and acute effectiveness of pulmonary vein isolation (PVI) with the third-generation cryoballoon catheter. No differences were observed in acute effectiveness, total procedure time, freeze time, need for additional applications and complications in groups with lower (OI < 1.27) and higher (OI > 1.27) OI values. In the analyzed population, increased pulmonary vein OI had no negative effect on the cryoballoon ablation (CBA) procedure performed with the third-generation cryoballoon catheter. Therefore it can be considered as a more comprehensive single-shot PVI tool.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, significantly increasing the risk of stroke and all-cause mortality [1]. Moreover, it is associated with increased risk of cognitive decline and early-onset dementia [2]. Pulmonary vein isolation (PVI), either with point-by-point radiofrequency ablation (RFA) or cryoballoon ablation (CBA), is the most effective AF treatment [3]. CBA has become a widely accepted PVI technique in the last decade. Randomized clinical trials proved that both the effectiveness and safety of RFA and CBA are comparable [4]. Furthermore, CBA using the second-generation cryoballoon leads to greater durability of PVI, shortening of procedure time and reduction of complications, also with the sequential use of this single-shot technique [5–9]. Moreover, the results of two recently published trials, STOP AF First and EARLY-AF, proved high effectiveness of CBA as the initial AF therapy [10, 11]. Optimal circumferential contact between the cryoballoon and the PV ostium is crucial for complete vein occlusion to achieve acute PVI and long-term freedom from AF [12]. Currently, one size of cryoablation balloon is used (28 mm), and the pulmonary vein (PV) anatomy may be very heterogeneous. There are numerous studies evaluating the impact of PV geometry, including ostium area, ovality, angulation and possible presence of a common trunk on the efficacy of CBA [13–18]. However, there are limited data concerning the association between PV anatomy and procedural parameters during CBA, all of them reporting the effects of first- or second-generation cryoballoon catheters. The crucial difference between the second and third cryoballoon generation is 40% shorter length of the distal tip (13.5 vs. 8 mm), which facilitates maneuvers and positioning of the balloon in the PV ostia [19]. This can possibly result in more stable catheter placement and influence the cryoapplication parameters.
Aim
In the current study, we aimed to investigate relationship between the ovality index (OI) of pulmonary vein ostia and procedural parameters such as nadir temperatures, procedure and fluoroscopy time and freeze duration per vein during the CBA procedure performed with the third-generation cryoballoon.
Material and methods
Study population
We retrospectively analyzed fifty-four consecutive patients (34 male; age: 62.2 ±11.3 years) with documented drug-refractory, symptomatic paroxysmal (77.8%) or persistent (22.2%) atrial fibrillation, who underwent PVI with third-generation cryoballoon ablation between March 2019 and July 2020 at our electrophysiology laboratory. In the analyzed group, hypertension (77.8%) and diabetes mellitus (18.5%) were the most common concomitant diseases. The studied population had substantial risk of thromboembolic complications (CHA2DS2-VASc score = 2.2). All analyzed patients were retrospectively assigned to one of the two groups (depending on the OI value as assessed with computed tomography (CT) scan described in detail in section 2.2). We decided to take the mean OI of all pulmonary veins in each individual as the most comprehensive parameter. Considering the fact that only 63% of the studied patients presented typical four-vein pulmonary drainage, which is consistent with the observations on large cohorts [20], and that the intention of the study was to evaluate the real-life setting, the left common PV ostium and supernumerary veins, if present, were also included in the calculation. Baseline characteristics of the studied group are presented in Table I. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki; it was approved by institutional review board and written informed consent was given by all participants.
Table I
Comparison of baseline characteristics between the groups of patients with low and high OI
Computed tomography imaging
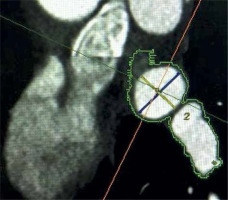
All patients were scanned on a 64-detector row CT scanner (Revolution EVO; GE, Milwaukee, WI, USA). CT scan parameters included: detector collimation 64 × 0.625 mm, tube voltage 120 kVp, tube current 215–480 mAs/rotation, pitch factor 0.16 mm/rotation, gantry rotation 0.35 s. Electrocardiographic gating was performed to minimize cardiac motion artifacts. All scans were performed in a cranial to caudal direction, starting immediately above the tracheal bifurcation to the level of the inferior margin of the cardiac apex. Images were constructed starting from early systole (10% of R-R interval) and ending at end-diastole (90% of R-R interval) using 10% steps. Reconstructed image data were transferred to a remote workstation (Advantage Windows 4.3, GE, St. Giles, UK) for post-processing. An 70–80% phase location corresponding to atrial end-diastole was selected for image reconstruction. If the patients had ongoing AF at the time of the CT scan, a 50% phase location was selected for image reconstruction to obtain the best image quality. In each patient left atrial volume, the PV drainage pattern according to Marom [21], the numbers of PV ostia, type of LA appendage and the presence of left atrial thrombosis were evaluated. Multi-slice cardiac computed tomographic studies were primarily viewed in the transverse, coronal and in the coronal-oblique plane. Using a dedicated automatic cardiac program, which included the oblique-vertical long axis plane, oblique long axis plane and oblique short axis plane, the optimal oblique sagittal view on the ostium was generated, used subsequently to measure its diameters. The PV ostium was defined as the visual anatomical entry point of the PVs in the atrial wall [21]. Endoluminal views were routinely rendered, allowing precise visualization of the pulmonary veins’ ostia. Left atrial 3D representations were helpful to evaluate the number of veins. Additional pulmonary vein was defined as an extra PV up to the pattern of 4 PVs. A right supernumerary vein was qualified as a separate ostium for the middle pulmonary vein lobe [22]. Left common pulmonary vein was defined as the presence of bifurcated PVs entering the left atrial contour together and a distance between the virtual border of the atrium and the bifurcation of both PVs ≥ 5 mm [23]. The OI was defined as the ratio of the maximum (Dmax) and minimum (Dmin) diameter of each PV ostium and was calculated using the formula Dmax/Dmin (Figure 1). In patients whose quality of examination allowed it, coronary arteries were additionally evaluated.
Periprocedural management
All patients were on oral anticoagulation at least 4 weeks before the procedure. For vitamin K antagonists (VKAs), an uninterrupted anticoagulation strategy was used, with the therapeutic international normalized ratio (INR) value during the ablation procedure. For novel oral anticoagulants (NOACs), we accepted an interrupted dosage scheme, with the preceding dose 12 h (dabigatran/apixaban) to 24 h (rivaroxaban) before, and the following dose 6 h after the ablation. In all patients, pre-procedural transesophageal echocardiography (TEE) was performed to exclude the presence of left atrial thrombus.
Cryoballoon ablation procedure
All cryoballoon ablation procedures were performed under conscious sedation with local anesthesia as described before [24]. In brief, after two punctures in the right femoral vein a steerable quadripolar or decapolar diagnostic catheter (Inquiry, Abbott, Minneapolis, MN, US) was introduced into the coronary sinus (CS). After a single transseptal puncture (BRK-1 needle, Abbott, St. Paul, MN, US) performed under fluoroscopic guidance, a 28-mm cryoballoon (AF Advance ST, Medtronic, Minneapolis, Minnesota, US) was introduced into the left atrium using a steerable sheath (FlexCath, Medtronic, MN, US). Before the puncture, a loading dose of 2500 IU of heparin was given, followed by an additional weight-dependent dose to reach 70 IU/kg and target activated clotting time (ACT) > 300 s. The cryoballoon was positioned against the PV ostia over the inner lumen mapping catheter (Achieve, Medtronic, Minneapolis, MN, US) introduced into the vein. After proper occlusion of each vein was confirmed with contrast injection, cryoapplication was commenced. The application sequence was left superior PV (LSPV) – left inferior PV (LIPV) – right superior PV (RSPV) – right inferior PV (RIPV). If a temperature of – 40°C or below was achieved at 60 s, cryoapplication time of 180 s was selected; otherwise it was 240 s [25]. In order to avoid phrenic nerve palsy, diaphragmatic stimulation from the right subclavian vein was performed during right-sided cryoapplications. The application was immediately interrupted in case of decreasing or loss of diaphragmatic response [26]. Ablation was also terminated if the temperature dropped to –60°C [27]. Bidirectional electrical PVI was confirmed using the inner lumen circular mapping catheter. Bidirectional (or unidirectional in case of ongoing atrial arrhythmia) electrical isolation of all pulmonary veins was considered as the endpoint of the cryoablation procedure. Directly after the procedure, transthoracic echocardiography was performed in all patients to exclude pericardial effusion.
Statistical analysis
Statistical analysis was performed with suitable statistical tests and Statistica software (StatSoft, Tulsa, OK, US). The values were presented as means and standard deviations (SD), medians, minimum and maximum value, and the range of variability. Normal distribution of continuous variables was tested with the Shapiro-Wilk test. Student’s t-test or the Mann-Whitney U-test for independent variables was used for intergroup comparison. The distribution of discrete variables in groups was compared with Pearson’s χ2 test or Fisher’s exact test. The error was set at 5% and a p-value < 0.05 was considered significant.
Results
Computed tomography imaging of pulmonary vein anatomy
A total of 54 consecutive procedures with complete CT scan data were analyzed. In the studied population, in 20 out of 54 (37%) patients atypical venous drainage pattern was present. The left common pulmonary vein was observed in 11 (20.4%) patients, and 9 (16.7%) patients had right additional pulmonary veins. Right-sided PVs were more circular (mean OI = 1.23) compared to left-sided (mean OI = 1.33). In the studied population, the LIPV was the most oval vein (mean OI = 1.42), followed by the RSPV (mean OI = 1.32), LCPV (mean OI = 1.31), LSPV (mean OI = 1.28), RIPV (mean OI = 1.2) and right middle pulmonary vein (RMPV) (mean OI = 1.16) as the most circular, when present. Based on the mean OI calculated for all pulmonary veins in each individual, the median OI of 1.27 was calculated for the whole group of 54 patients, and subsequently the study population was divided into two equal groups: Group 1 with a lower OI (OI < 1.27) and Group 2 with a higher OI (OI ≥ 1.27).
CBA procedural parameters, acute success and complication rate
Procedural parameters, acute success and complication rate were compared between Group 1 and Group 2. An acute effect assessed as post-procedural PVI was achieved in all ablated patients in both groups. There were no differences in total skin-to-skin procedure time (81.0 ±19.0 min vs. 81.3 ±21.3 min), total freeze time (899.6 ±233.0 s vs. 849.7 ±148.4 s), need for additional applications (11/27 vs. 8/27) and complication rate (3/27 vs. 5/27). There was a slight difference in the fluoroscopy time, which was longer in patients with a lower OI (12.6 ±4.3 min vs. 10.4 ±3.4 min; p = 0.046), however there was no significant correlation calculated between the mean OI and any of the procedural parameters (Tables II and III). Moreover, we analyzed the correlation between OI and procedural parameters for each specific vein separately: freeze time, temperature drop at 60 s of freeze (T60s), the lowest temperature reached (Tnadir) and thawing time to reach 0°C (Tt0°). There was a trend towards a lower absolute value of Tnadir and shorter thawing time in more oval left-sided common pulmonary veins (LCPVs) as well as shorter freeze time in more oval left inferior PVs; however, the differences were not statistically significant (Table IV). In fact, the parameters achieved in the most oval PV ostium (OI = 2.42) were as follows: T60 – 45°C, Tnadir – 51°C and freeze time 180 s. Acute electrical isolation was achieved in 100% of PVs irrespectively of OI value or complex anatomy. There were no major complications of the procedure. Minor complications (with no difference between the groups, p = 0.7) included transient phrenic nerve palsy, resolving before the end of the procedure, and minor groin hematoma.
Table II
Comparison of procedural parameters between the groups of patients with low and high OI
Table III
Correlation between OI and procedural parameters
| Parameter | Procedure time [min] | Total freeze time [min] | Fluoroscopy time [min] |
|---|---|---|---|
| Mean ovality index | R = 0.043 p = 0.76 | R = –0.055 p = 0.69 | R = 0.257 p = 0.06 |
Table IV
Correlation between OI and procedural parameters in each pulmonary vein
Discussion
To our knowledge, this is the first study evaluating the impact of the pulmonary veins’ anatomy on cooling kinetics and the acute effect during AF ablation with the third-generation cryoballoon catheter. In the published literature, regarding the effects of PVI with the second-generation cryoballoon catheter, the authors postulated that an optimal and stable circumferential cryoballoon contact to the PV ostium is crucial to achieve optimal temperatures and to create a durable lesion [28–32]. Schmidt et al. reported that a high PV OI impedes durable lesion formation [29], but it is feasible to perform CBA even in a patient with extremely flat pulmonary veins [30]. The relationship between OI and cryoballoon adhesion has also been reported by Sorgente et al. [23], who demonstrated that the OI of left-sided PVs is associated with the degree of occlusion. In their analyzed population, left-sided PVs were more oval, which is consistent with our data. Additionally, they found a strong association with the PV orientation on the frontal plane [23]. There are also reports on the impact of pulmonary vein anatomy on AF recurrence, demonstrating that area of pulmonary vein ostium [13], shape of the carina between PVs and between the PV and the LA appendage, early branching and nonperpendicular orientation of the RIPV [32] and likewise angulation [32] were predictors of acute and mid-term CBA failure. Another report demonstrates that ventral-caudal orientation of both the LSPV and LIPV is significantly associated with AF recurrence [16]. Contrarily, Mulder et al. observed no specific characteristics of PV dimensions or morphology associated with AF recurrence after cryoablation [33]. Only a few recent studies investigated the association between PV anatomy and procedural cooling kinetics during CBA. Borio et al. confirmed that PV diameters, area of the ostium, ovality and trunk length were associated with temperatures achieved during CBA. They emphasize the cumulative role of these parameters (put together in a score) to predict satisfactory cryokinetics [34]. Some of these observations were confirmed in the study published by Chen et al., who reported an association between the lowest temperature achieved (Tnadir) and PV ostial diameter, but not shape [35]. Kajiyma et al. reported that both the nadir balloon temperature and temperature at the starting point of the slow cooling phase were significantly associated with the area of the pulmonary vein ostium; additionally, in their analysis a higher OI was predictive for a higher number of cryoapplications needed to achieve acute PVI [13]. Remarkably, in the reviewed publications CT scanning was a common imaging tool used for the PV anatomy assessment, even though considering the results of 1STOP study [36], cryoballoon is effective regardless of the availability of the pre-procedural CT imaging data. On the other hand, with challenging anatomy, some supernumerary veins, particularly with uncommonly located ostia, can easily be omitted during the ablation procedure, leaving potential AF triggers non-isolated. Therefore, the evaluation of PV anatomy appears reasonable to adequately plan the procedure. Regarding the correlation between CT imaging and transesophageal echocardiography, TEE-based pre-procedural PV assessment if feasible; however, it again can be challenging with clear identification of anatomical variants [37].
In our study, we compared: total procedure time, fluoroscopy time, freeze time and the need for additional applications in patients with low and high mean OI values. Moreover, we evaluated the impact of PV’s OI on procedural cooling kinetics during third-generation cryoballoon ablation. There was no correlation between the OI and any of the analyzed parameters, nor the difference in the procedural timing between the two groups with high and low OI values, except for surprisingly slightly shorter fluoroscopy time in the group with more oval veins. Logically, with the potentially easier occlusion of the rounder pulmonary veins, shorter procedure and fluoroscopy duration should be anticipated. This paradoxical finding can be explained by slightly more frequent atypical pulmonary veins geometry in low OI group. In Group 1, although equally populated, the total number of the targeted veins was higher, which, still being below the significance threshold, resulted in a slightly longer fluoroscopy time. Therefore, it seemed not to be directly associated with ovality of the veins. Theoretically, an incomplete occlusion should result in a higher balloon temperature, because the remaining PV blood flow has a rewarming effect on the cryoballoon [38]. Apparently, the more oval the PV ostium, the more difficult it is to obtain optimal balloon adhesion. However, both the PV ostia and the cryoballoon itself are compliant to some extent, and with the mutual conformation it is possible to achieve complete closure of even an extremely flat vessel. Moreover, cooling kinetics depend not merely on the degree of balloon adhesion. The thermocouple of the CB is located in the proximal part of the balloon and recorded temperatures do not precisely reflect the temperatures reached in the ablated tissue. Cryoballoon rewarming may be caused not only by PV to LA blood flow but additional factors may influence the kinetics. One of them is position of the thermocouple in the LA; in particular, the closer to the mitral valve and the more central the LA position, the higher is the degree of blood flow rewarming. Importantly, all procedures analyzed in our study were performed using third-generation CB, while the previous reports refer to the first- and second-generation CB. Our findings may suggest different thermal dynamics between consecutive generations of cryoballoon catheters. Furthermore, we confirmed high safety of the CBA procedure, with the overall minor complication rate similar to the reports by other authors [24–27], with no difference between the groups.
There are several limitations of our study. Firstly, it is a single-center, observational study, performed on a relatively small number of patients, and therefore with limited statistical power. Secondly, since all patients with supernumerary veins and/or common ostia were included to maintain the real-life settings scenario, the uneven distribution of accessory pulmonary veins between both groups, although statistically non-significant, could possibly influence the results. Moreover, the calculated median OI in the studied group does not necessarily represent the median OI for the general AF population, which was the rationale for additional (not median-related) analysis of the correlation between the OI and procedural parameters, as well as the correlation for each specific PV separately. And last, the slight trend towards better clinical outcome in the patients with lower OI that was observed in mid-term follow-up may indicate that, considering the influence of PV ostia anatomy on the cryoballoon ablation outcome, there is possibly more to it than just the cryokinetic parameters we can assess.
Conclusions
In the studied population increased pulmonary vein OI had no negative effect on the CBA procedure performed with the third-generation cryoballoon catheter. Further, larger studies with long-term follow-up are needed to advocate the use of the third-generation cryoballoon as a more comprehensive single-shot tool for pulmonary vein isolation.