Introduction
Infective endocarditis (IE) on the tricuspid valve is associated with IE on other valves in up to 90% of cases. Isolated IE on the tricuspid valve involves only 1% to 10% of all IE cases [1, 2].
The most common cause of tricuspid IE is intravenous contamination of the valve in intravenous drug abusers and in patients undergoing chronic intravenous therapy, or as the result of pacemaker and electrode infection [3].
The aim of the surgical procedure is to eliminate the infection, prevent further valve destruction, improve cardiac hemodynamic function, and take material for microbiological tests which enables modification of antibiotic therapy.
The indication for surgery for tricuspid valve IE is severe regurgitation causing heart failure or echocardiographic features of uncontrolled infection (abscess, fistula, enlarging vegetation). Infections caused by fungi, opportunistic microorganisms, staphylococci, or non-HACEK Gram-negative bacteria with persistently positive blood cultures despite appropriate antibiotic therapy are also an indication for surgical intervention [2, 4]. The risk of embolization in decision making concerning surgery is less important than in left heart valve endocarditis because of fewer clinical implications.
There are no broader data supporting the choice of a specific surgical treatment in this patient population. The elimination of the infection requires the complete resection of the infected tissue, which usually means resection of the entire native valve and implantation of a valve prosthesis. In rare cases, valvular reconstruction is possible, e.g. when the infection is limited to only a part of the leaflet which could be removed or replaced with a pericardial patch. In cases of infection limited to the posterior leaflet, it can be completely resected followed by valve bicuspidalization. In extreme cases of recurrent IE in drug addicts, complete resection of the infected valve without implantation of a prosthesis is performed.
If valve replacement is necessary the majority of authors prefer the biological prosthesis, only a few the mechanical [5]. However, any prostheses in the tricuspid position are particularly sensitive to recurrent infection, which carries a four-fold increase of operative risk in case of reoperation [2, 6]. Moreover, prosthetic valves are at risk of either rapid degeneration (bioprostheses) or thromboembolism (mechanical valves). Tricuspid valve replacement or even a ring implantation is associated with insertion of artificial material into the infected area and they carry a significant risk of recurrent infection ranging from 8% to 21% [7, 8].
Aim
We assumed that total elimination of artificial material and implantation of an entirely patient-derived biological material would reduce the recurrence of IE. There is no prosthesis designed exclusively for tricuspid orifice anatomy.
Therefore, we used the method of implanting a pericardial cylinder created from pericardium into the tricuspid orifice.
Material and methods
Material
The group consisted of 7 consecutive patients operated on between August 2021 and April 2022. There were only men aged 43 to 73 years. In 5 patients positive blood cultures were obtained with the growth of various types of pathogens (Table I). In 2 patients, blood cultures were negative and the decision to perform surgery was based on echocardiographic findings. Two (29%) patients were active drug users.
Table I
General information about patients
Methods
The decision to implant a pericardial cylinder in the tricuspid position was made in patients in whom, because of total destruction of the tricuspid leaflets, valve replacement was the only therapeutic alternative.
Transthoracic echocardiogram (TTE) was used to assess the extent of leaflet destruction, degree of regurgitation, and annular size, and estimate the cylinder valve height (distance from the annular margin to the papillary muscle heads).
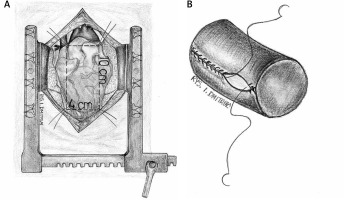
Operations were performed under general anesthesia. Median sternotomy was performed, which is necessary to take pericardial material. A rectangular patch of pericardium 10 × 4 cm was taken. After anastomosing the shorter sides of the patch with continuous Prolene 5-0 suture, a cylinder of 30–32 mm diameter was created. The length of the cylinder was approximately 120% of its diameter [3]. Three points every 120° were marked on the cylinder as the expected attachment points inside the right ventricle. In 2 patients, the cylinder was made from a bovine pericardial patch (Edwards Lifesciences, model: 4700) in the same fashion. The tissue cylinder was prepared prior to starting the ECC (Figure 1).
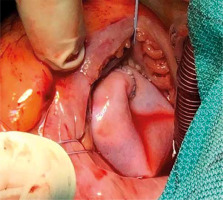
Operations were performed with the use of ECC with separated venous drainage. After clamping the aorta, cold blood cardioplegia was administered to the aortic root and repeated every 20 minutes. The right atrium was opened and tricuspid valve was excised completely, together with all infected tissue. The heads of the papillary muscle were identified. The previously marked 3 points of the pericardial cylinder were attached to the heads of the posterior and septal papillary muscles using Gore-Tex 5/0 sutures. If the anterior papillary muscle was poorly developed, the corresponding point of the cylinder was attached to the interventricular septum at the level of the other papillary muscle heads. The proximal part of the cylinder was anastomosed to the annulus of the tricuspid valve using continuous Prolene 5-0 suture, gradually compensating the difference in diameter between the native annulus and cylinder. After implantation, a water test was performed to confirm the tightness of the created valve (Figure 2).
The function of the implanted valve was examined after weaning from ECC by transesophageal echocardiogram (TEE), followed by TTE on days 5–7 after surgery.
The postoperative follow-up period ranged from 2 to 32 months (median 17 months). The follow-up was performed in the outpatient department (4 patients) or via telephone interview (1 patient).
Isolated implantation of the pericardial cylinder was performed in 2 patients. Five patients needed additional procedures (implantation of prosthesis in other orifices, atrial fibrillation ablation, ventricular septal defect (VSD) closure, patent foramen ovale (PFO) closure, coronary artery bypass grafting (CABG)) (Table I).
Results
Operative time
In 2 patients who underwent isolated tissue cylinder implantation, the average extracorporeal circulation (ECC) time was 77.5 minutes and aortic cross-clamp time was 58 minutes. In 5 (71%) cases additional procedures were performed. The ECC and X-clamp times in this group were 197.4 and 156.2 minutes, respectively (Table II).
Table II
Characteristics of surgery and follow-up
Echocardiographic findings
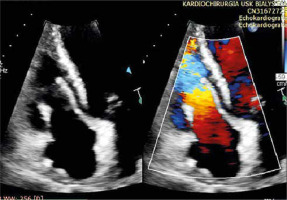
The transesophageal intraoperative examination revealed in all cases the normal morphology and function of the implanted pericardial cylinders in terms of regurgitation, leaflet mobility, coaptation height, and orifice area. A TTE (Figure 3) performed 1 week after surgery confirmed the normal function of the prosthesis in all patients.
Postoperative complications and deaths
Postoperative complications occurred in 3 cases (Table II). Death occurred in 2 patients. One patient (patient 4) was admitted for surgery in a critical condition and developed postoperative renal failure, acute pancreatitis, and gastrointestinal bleeding. This patient died in the Intensive Care Unit (ICU) on the 139th day without leaving the hospital. The second patient (patient 1) died 5 months after surgery (Table II).
Recurrence of IE
In the follow-up period none of the patients had a recurrence of IE within the pericardial cylinder implanted in the tricuspid orifice.
In the entire group, in addition to pericardial cylinder implantation in the tricuspid position, 4 patients had prosthetic valves implanted in other positions because of coexisting IE (Table I). Two of these patients had a recurrence (or suspected recurrence) of IE. In none of these cases was the microorganism from the first infection repeated. One of them (patient 1), a patient with a history of active drug abuse, developed a recurrence of IE after 5 months within the Perimount 23 bioprosthesis implanted in the aortic position with presence of a perivalvular abscess. The tissue cylinder implanted in the tricuspid position did not show echocardiographic signs of recurrent IE. The patient was admitted to the ICU in an extremely severe condition, with third degree AV block and ST elevation myocardial infarction (STEMI). Despite intensive treatment the patient died without any improvement. In the second patient (patient 3), in the peripheral hospital recurrence of IE was suspected on the mitral and aortic mechanical prostheses. Diagnosis was based on one positive blood culture and echocardiographic examination (perivalvular aortic abscess and vegetation in the area of mitral prosthesis). No recurrence of IE within the tricuspid valve cylinder was suspected. Antibiotic therapy was initiated, and the patient was transferred to our department. After admission, no positive culture was obtained, and TTE and TEE examination did not show recurrence of IE in any site. Nevertheless, antibiotic therapy was continued with rifampicin (for up to 16 days) and vancomycin (for up to 38 days). The patient in good condition was discharged home with continuation of oral antibiotic therapy for another 4 weeks. Over the next 4 months, the patient had recurrent episodes of fever with negative blood cultures or other signs of IE, which have spontaneously resolved.
Pericardial cylinder durability
Degeneration with subsequent stenosis of the pericardial cylinder occurred in 3 patients (patients 1, 2, 6) (Table II). Two had a history of drug addiction. One of them (the aforementioned patient 1) developed cylinder degeneration and died 5 months after surgery in the course of recurrent IE on the prosthetic aortic valve. The second drug addicted patient (patient 6) developed early cylinder degeneration 3 months after surgery.
One non-addicted patient (patient 2) developed cylinder stenosis 6 months after surgery. It was a patient with a history of pulmonary embolism, after a multi-site burn > 30% body surface area (BSA) including the oral cavity, esophagus and respiratory tract.
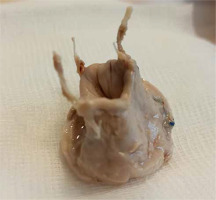
Both patients with cylinder degeneration (patients 2 and 6) were readmitted in severe condition with features of right ventricular failure (Table II). In both cases, urgent valvular balloon valvuloplasty was performed, which led to an immediate clinical improvement. The first patient underwent elective reoperation with removal of the pericardial cylinder followed by implantation of an Edwards Perimount Plus 31 mm stent bioprosthesis and closure of the PFO with a bovine pericardial patch (SJM Pericardial Patch with EnCap Technology). The removed degenerated pericardial cylinder morphologically appeared to be a durable structure (Figure 4). Therefore, in the next case, a transcatheter valve-in-valve procedure (Edwards Sapien 3 29 implantation) was performed into the fibrotic pericardial cylinder (Table II, Figure 4). To our knowledge this is the first reported case of Valve-in-pericardial cylinder Valve procedure.
During the follow-up period, no evidence of pericardial cylinder degeneration was found in the remaining patients.
Discussion
Substitution of artificial material with autologous tissue in the surgical treatment of IE is considered to be the procedure of choice. Autologous tissue, or tissue harvested from and used for the same patient, is the current gold standard on account of its superior functionality and nonimmunogenicity. Supply of tissues needed and health status of the patient are major hindrances to obtain autologous tissue [9]. The choice of native tissue was related to the fact that it is the material most resistant to infection. Even biological valves are largely made of artificial material, which affects the risk of recurrence of IE [4, 10]. IE of heart valves usually required valve replacement. Cox’s concept of the construction of a cylindrical valve made of fully biological material was first tested in the aortic position [11]. Wallen and Rao used a cylinder from biological material in the tricuspid position, which became the basis for the wider use of this method [12]. Such procedures using CorMatrix material are performed in children due to the growth potential of such a valve [13].
To our knowledge, it is the largest single center material of implantation of a tissue cylinder in the tricuspid orifice in the adults. In a Canadian study, the largest multicenter study to date, similar operations were performed on 18 patients [3]. In their study 4 mechanical complications occurred due to ruptures of the attachment of the cylinder to the papillary muscles, or the cylinder itself. In our material, such complications did not occur. This may be related to different technique and different materials. In the Canadian study, small intestinal submucosa (CorMatrix) was used as the material for construction of the cylinder, and polypropylene suture was used for the attachment to the papillary muscles. We used patients’ own or bovine pericardium to construct the cylinder, and for attachment to the papillary muscles we used Gore-Tex 5/0 suture. In addition, unlike the Canadian work, the operations were performed by 2 and not by 12 surgeons (Figures 5 A, B).
We hypothesized that the use of the biological cylinder from the patient’s own pericardium might be the best surgical solution in patients with tricuspid IE. Indeed, there was no recurrence of IE on the tricuspid valve pericardial cylinder. In all cases, with different pathogenic microorganisms, we have successfully treated IE of the tricuspid valve. This did not apply to commercial valves implanted in other positions as additional procedures.
The success of eliminating the recurrence of IE after pericardial cylinder implantation in the tricuspid orifice did not translate into durability of this valve. In 3 cases, rapid degeneration and stenosis of this valve were observed within 6 months after the surgery. A possible reason may be that we used the patient’s pericardium not fixed in glutaraldehyde. We considered that patients’ living tissue would be most resistant to infection in an infected environment. No early degeneration was observed in the remaining patients with the cylinder constructed from their own pericardium, and in 2 cases of the cylinder made from bovine pericardium.
In 1 case of a stenotic cylinder, a classical reoperation was performed, and a commercial bioprosthesis was implanted. In the setting of an uninfected surgical field, such re-do surgery resulted in a good outcome. Since the removed degenerated cylinder macroscopically appeared to be a durable structure, in the second case we decided to perform a valve-in-valve transcatheter valve implantation. It was the world’s first transcatheter valve implantation into the pericardial cylinder in the tricuspid position. During the follow-up, both patients were asymptomatic and showed proper function of valvular prostheses.
The observed rapid degeneration of the pericardial cylinder from the patient’s own pericardium is an unfavorable event that requires reoperation with possible subsequent complications. Perhaps fixation of the pericardial tissue in glutaraldehyde would bring better results in terms of durability of the pericardial cylinder. On the other hand, the good outcomes of implanted cylinders prepared from bovine pericardium may suggest that this biological material should be considered for the procedure. The problem of tissue selection and preparation for cylinder construction requires further study.
Conclusions
Implantation of the pericardial cylinder in the tricuspid position effectively eliminates IE of the tricuspid valve and the risk of IE recurrence. Implantation of a patient’s pericardial or bovine pericardial cylinder into the tricuspid position restores valve function and is an effective and reproducible method. Cylinder degeneration with subsequent stenosis could be treated with balloon valvuloplasty and transcatheter valve-in-valve implantation.