Introduction
According to the American Cancer Society, bladder cancer (BC) is the most common cancer in the urinary system [1]. In 2024, it was estimated that there would be approximately 83,190 new cases and 16,840 new deaths due to this disease within the United States [2]. BC clinically presents with visible hematuria, and its diagnosis and staging are facilitated by a combination of cystoscopy and imaging studies, such as pelvic magnetic resonance imaging [3]. These studies help in classifying the cancer into non-muscle- invasive BC and muscle-invasive BC (MIBC) [4]. Patients with MIBC often undergo radical cystectomy or receive radiosensitization combined with concurrent chemotherapy or immunotherapy using checkpoint inhibitors. These treatments can result in significant discomfort and impose a substantial financial burden [5]. Patients with metastatic disease typically have a median survival of approximately 13 to 15 months when treated with standard chemotherapy [3]. It is concerning that the pathogenesis of BC remains incompletely understood, and current treatments are not yet capable of blocking the progression of the disease.
Since the discovery of the Warburg effect, we have become increasingly aware that cancer metabolism differs significantly from that of normal cells, primarily due to the functioning of oncogenes and tumor suppressor genes [6]. Cancer-specific metabolic pathways or enzymes often serve as therapeutic targets for the development of antitumor drugs [7]. Researchers found that metformin suppressed BC cell proliferation by targeting the glycoprotein clusterin, thereby regulating lipogenesis through the SREBP-1c/FASN signaling axis [8]. It was concluded that glycerol 3-phosphate dehydrogenase 1 facilitated calcium influx and enhanced apoptosis in BC cells, thereby suppressing their growth through the lysoPC-PAFR-TRPV2 pathway [9]. Bo Xie and colleagues [10] concluded that CircXRN2, a newly identified therapeutic target for human bladder cancer, curbs tumor progression by activating the Hippo pathway, thereby promoting H3K18 lactylation and LCN2 expression. The metabolic functions in BC that merit deeper investigation offer promising avenues for developing novel therapeutic strategies.
Mendelian randomization (MR) leverages instrumental variables (IVs) derived from Genome-Wide Association Studies (GWAS) to investigate the causal relationship between exposures and outcomes [11]. Researchers conducted MR analysis, which suggested that telomere shortening, potentially through pathways associated with cellular senescence, may drive the development of idiopathic pulmonary fibrosis [12]. Cornish et al. [13] applied MR techniques to rigorously assess the causality and magnitude of the association between 39 putative modifiable risk factors and the risk of colorectal cancer. By performing integrated MR analysis, we investigated potential risk genes associated with BC within the apoptosis-related gene set, along with their underlying metabolic mechanisms, aiming to identify novel therapeutic targets.
Material and methods
Study design and data sources
A comprehensive flowchart of our study is depicted in Figure 1. The IVs were rigorously selected based on three MR basic criteria: they must be correlated with the exposures, free from confounding influences on the outcomes, and exert their effect on the outcomes exclusively through the exposures [14]. This study adhered strictly to the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology-Mendelian Randomization (STROBE-MR) initiative [15].
Figure 1
Flowchart of our study, which aimed to explore the interrelationships among apoptosis-related genes, metabolites, and BC
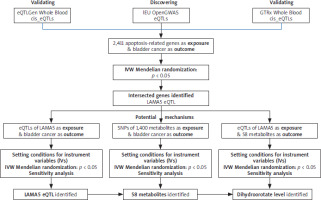
Our study used a list of 2,411 apoptosis-related genes as the exposures, which was derived from a prior publication [16]. The expression quantitative trait loci (eQTLs) data for these genes were freely accessed in the IEU Open-GWAS database (https://gwas.mrcieu.ac.uk/). The dataset of metabolites was derived from a comprehensive series of publicly accessible GWAS, encompassing a total of 1,091 distinct metabolites and 309 metabolite ratios [17]. Genetic data pertaining to BC, serving as the outcome variable, were also extracted from the IEU OpenGWAS project, specifically using the dataset identified by the ID ieu-b-4874, which encompasses 1,279 cases of European ancestry publicly.
Selection of IVs
We established distinct sets of stringent genetic instrument standards for each two-sample MR analysis to ensure the validity and robustness of causal inferences. When considering apoptosis-related genes as the exposures and metabolites or BC as the outcomes, we selected single nucleotide polymorphisms (SNPs) strongly correlated with the exposure, using a stringent filter criterion of p < 5 × e–8. To ensure independence from linkage disequilibrium, we applied admission standards with r2 = 0.001 and kb = 10,000 kb. Additionally, we eliminated weak instrumental variables by applying an F statistic threshold > 10. In our study with metabolites as the exposures and BC as the outcome, we instituted stringent criteria for SNP inclusion, requiring genome-wide significance (p < 1 × e–5), minimal linkage disequilibrium (r2 < 0.001 within a 10,000 kb window), and strong instrumental variable properties (F-statistics > 10), to ensure the robustness and reliability of genetic associations.
Two-sample MR analysis
In this study, we performed three separate two-sample Mendelian randomization (MR) analyses. Initially, we identified 2,411 apoptosis-related genes and 1,400 metabolites separately as exposure sets, with BC selected as the outcome in each analysis. Subsequently, we focused on the genes that exhibited a statistically significant association with BC as the exposure and examined 58 selected metabolites as potential outcomes to accurately identify the metabolite with the strongest associations for further in-depth investigation.
We employed a diverse array of five distinct analytical methods to investigate the relationship between exposures and outcomes. Each of these methods generated scatter plots that incorporated the IVs. The primary technique was the inverse variance-weighted MR (MR-IVW), complemented by MR-Egger, weighted median, simple mode and weighted mode as additional techniques. The IVW method is the most established and fundamental MR approach, leveraging a comprehensive meta-analytical framework to synthesize the Wald estimates derived from each individual genetic variants [18]. We performed pleiotropy testing using the Egger-intercept method and assessed the heterogeneity through Cochran’s Q test for IVW and Rucker’s Q test for MR-Egger [19]. Additionally, we conducted leave-one-out analysis to ensure the robustness of our findings [20].
SMR analysis and heterogeneity in dependent instruments (HEIDI) test
In our SMR analysis, multiple SNPs associated with the exposure at a cis-eQTL locus were used as IVs to validate the findings from our previous two-sample MR analysis, which investigated the association between apoptosis-related genes and BC. Cis-eQTLs are typically situated in promoter or enhancer regions and modulate the expression levels of proximal genes on the same chromosome by impacting their transcriptional activity [21]. The association was validated using cis-eQTL summary- level statistics derived from two distinct databases: the Genotype-Tissue Expression (GTEx) project and the eQTLGen Consortium. We conducted the HEIDI (Hetero-geneity in Dependent Instruments) test to minimize the potential for pleiotropy arising from linkage disequilibrium [22]. All results satisfied the criterion for statistical significance, with p-values less than 0.05.
Cell culture
The human bladder cancer cell lines T24, 5637, and RT112, procured from the Cell Bank of the Chinese Academy of Sciences, were cultured in RPMI 1640 medium (Gibco), while the immortalized human urothelial cell (SV-HUC-1) was cultured in F-12K medium (Gibco). The medium was enriched with 10% fetal bovine serum (FBS) sourced from Gibco and a combination of 100 mg/ml penicillin and streptomycin, procured from Sangon Biotech (Shanghai).
Quantitative real-time PCR (qRT-PCR)
Total RNA extracted from our cell lines was purified using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), followed by reverse transcription to synthesize cDNA. The master mix for the qPCR reaction consisted of 10 µL of SYBR Premix Ex Taq II (Takara), 2 µM each of the forward and reverse primers, 2 µl of cDNA template, and water to make up the final volume to 20 µl. The qRT-PCR was performed using the LightCycler 480 system (Roche) with the following thermal cycling conditions: an initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 10 s, 60°C for 45 s, and 72°C for 1 s. The primer sequences were: LAMA5 forward 5′-CCCACCGAGGACCTTTACTG-3′ and reverse, 5′-GGTGTGCCTTGTTGCTGTT-3′ and ACTB forward, 5’-GCC-GACAGGATGCAGAAGGAGATCA-3’ and reverse, 5’-AAGCA-TTTGCGGTGGACGATGGA-3’.
Cell transfection
T24 cells were initially seeded in a 6-well plate at a density of 2 × 105 cells per well and were allowed to grow until 60–80% confluence. Three distinct predesigned siRNA sequences were used to target the LAMA5 gene: siRNA1, GUGUGUGACCACUGUGUGGUC (sense) and CCACACAGUGGUCACACACUU (antisense); siRNA2, CUCGCCUCAUAGGUGUCUAUUTT (sense) and AAUAGACACCUAUGAGGCGAGTT (antisense); siRNA3, ACUGGAUCAGGCUGACUAUUUTT (sense) and AAAUAGUCAGCCUGAUCCAGUTT (antisense). According to the manufacturer’s guidelines for transfection reagents, transient transfections utilizing Lipofectamine 3000 (Thermo) were performed with a 50 nM concentration of each siRNA, which was diluted in Opti-MEM (Thermo). Furthermore, we included a negative control (NC) siRNA and a fluorescein-labeled siRNA to validate the knockdown efficiency. We replaced the transfection medium with complete medium 6 to 8 hours post-transfection. The transfection efficiency was verified by qRT-PCR at 48 to 72 hours post-transfection, after which the cells were harvested for subsequent analysis.
Cell viability assay
T24 cells were seeded into 96-well plates at 5,000 cells per well, with siRNA and NC groups, each with three replicate wells. When the cells adhered to the substrate, proliferation was evaluated using the CellTiter-Glo Luminescent Cell Viability Assay (Promega) following the protocol of the manufacturer. At the specified time points of 48 hours after treatment, 100 µl of reagent was precisely aliquoted into each well, and the plates were incubated for 10 minutes to allow for the luminescence assay. The luminescence (relative light unit, RLU) was taken for each well using a microplate reader to quantify the cellular proliferation.
Wound healing assay
NC and siRNA-transfected T24 cells were cultured into 6-well plates at a concentration of 6 × 104 cells per well, with triplicate wells for each group. Basal culture medium (4 ml) supplemented with 1% FBS was added to each well to establish the formation of a uniform cell monolayer. We used a 200 µl sterile plastic pipette to create a scratch in the cell monolayer, followed by the elution of suspended cells with PBS. Wound healing progress was photographed and observed at 48 hours post-scratch.
Transwell assay
Cell invasion assays were conducted using a 24-well Transwell chamber (Costar, Corning, NY, USA) precoated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). The NC and siRNA-transfected T24 cells were plated at a density of 6 × 104 cells per well. The upper chamber contained cells in serum-free and antibiotic-free medium, while the lower chamber was filled with complete growth medium. After 48 hours, cell fixation was performed using 95% methanol, followed by staining with crystal violet. Results were checked using an inverted microscope to capture images at the appropriate magnification, covering the top, middle, bottom, left, and right fields of view within the chamber.
Statistical analysis
All analyses were conducted using R software version 4.3.2 with the package “TwoSampleMR” version 0.5.8, SMR software, version 1.3.1, and GraphPad Prism software, version 8.0. Statistical significance was determined using a p-value threshold of less than 0.05 [11].
Results
The whole study design
The comprehensive flowchart of our study is depicted in Figure 1. Through the two-sample MR analysis between apoptosis-related genes and BC, we were interested in the role of LAMA5, while during the analysis between metabolites and BC, 58 metabolites remained to be further identified. Furthermore, we explored the association between LAMA5 and the chosen metabolites. Based on the initial two-sample MR analysis, we identified LAMA5 as the target gene and dihydroorotate levels as the selected metabolite for further discussion.
MR analysis between LAMA5 and BC
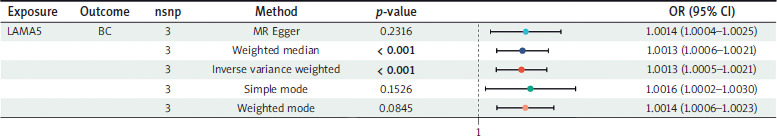
Three SNPs that met the criteria were used to investigate the association between LAMA5 and BC. As Figure 2 shows, all five methods employed to assess the two-sample MR effects consistently indicated a significant risk association of LAMA5 with BC. Regarding the results obtained from the IVW method, the odds ratio (OR) was calculated to be 1.0013, with a 95% CI ranging from 1.0005 to 1.0021. Additionally, the p-value is less than 0.001, indicating a statistically significant result. All the results successfully passed the tests for heterogeneity, pleiotropy, and leave-one-out analysis, thereby confirming their reliability.
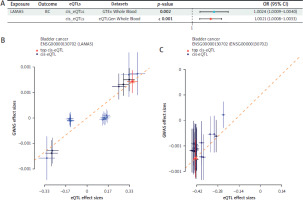
SMR analysis in other databases
Two separate databases were used to verify the rela-tionship between LAMA5 and BC. As Figure 3A reveals, the SMR analysis ultimately confirmed the association by meticulously selecting respective cis-eQTLs from the GTEx Whole Blood dataset (OR = 1.0024; 95% CI: 1.0009– 1.0040) and the eQTLGen Whole Blood dataset (OR, 1.0021; 95% CI: 1.0008–1.0033). The comprehensive results from both data-bases are depicted in Figures 3B and 3C through scatter plots, offering a detailed visual representation of the positive correlation. Both p-values were less than 0.005, and the HEIDI test was passed. In our analysis of the genetic link between LAMA5 and BC across the discovery dataset and two separate validation cohorts, we found consistent evidence that LAMA5 could be a risk factor for development of BC.
Figure 3
Results of the SMR analysis using eQTL data from two distinct databases, GTEx Whole Blood and eQTLGen Whole Blood. A) Forest plot for validation of causal relationships between LAMA5 and BC. B) Scatter plot for IVs chosen from GTEx Whole Blood. C) Scatter plot for eQTLs from eQTLGen Whole Blood
Expression of LAMA5 in BC cell lines
To investigate the expression of LAMA5, qRT-PCR was conducted across three human BC cell lines in addition to the immortalized human urothelial cells. As Figure 4A demonstrates, the expression of LAMA5 in all three cancer cell lines, T24, 5637, and RT112, was significantly high. To further explore the role of LAMA5 in BC, three different siRNAs were used to knock down the expression in the T24 cell line, and as depicted in Figure 4B, we finally chose sh LAMA5-3 to study further.
Figure 4
Outcomes of our validation studies conducted in BC cell lines. A) Expression of LAMA5 in three different human BC cell lines and immortalized human urothelial cells. B) Knockdown efficiency of three shLAMA5 compared with negative control siRNA. C) Luminescence of cell viability assay. D) Results of wound healing assay: images at 72 hours post-transfection, acquired using a light microscope and the histogram for area of wound healed. E) Results of invasion transwell assay: crystal violet stained images at 48 hours post-transfection obtained using a light microscope and the histogram for cell invasion rates
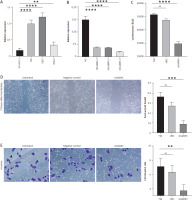
Knockout of LAMA5 inhibited BC cell proliferation in vitro
To further elucidate the role of LAMA5 in BC cell lines, we performed a cell assay to assess cell proliferation following a 48-hour transfection period. Consequently, the luminous intensity measurements demonstrated that T24 cells transfected with siLAMA5 exhibited a signifi-cantly reduced proliferation rate compared to both untreated cells and those transfected with siNC, as demonstrated in Figure 4C.
Knockout of LAMA5 suppressed BC migration and invasion in vitro
To evaluate the capacity of LAMA5 to modulate the migratory and invasive properties of bladder cancer cells, we employed both the wound healing assay and the transwell assay. The T24 cells were divided into three distinct groups: untreated controls, those treated with siNC, and those treated with siLAMA5. In the wound healing assays, cell density was monitored at 72 hours by microscopic evaluation, as shown in Figure 4D. In comparison to the control cells, the migratory activity, as indicated by the rate of wound closure, was significantly impaired by the knockdown of LAMA5. There was a notable difference in the migration activity between the siLAMA5 group and the other two groups, with the siNC group’s migration activity being comparable to that of the untreated cells. Consistently, the transwell invasion assays demonstrated that the knockdown of LAMA5 diminished the invasive capacity of BC cells. As illustrated in Figure 4E, this reduction was observed 48 hours after transfection and transwell assay. Additionally, significant variation in the rate of cell invasion was noted. Collectively, these findings suggested that LAMA5 knockdown might weaken the migratory and invasive capabilities of BC cells.
MR analysis between LAMA5 and dihydroorotate levels
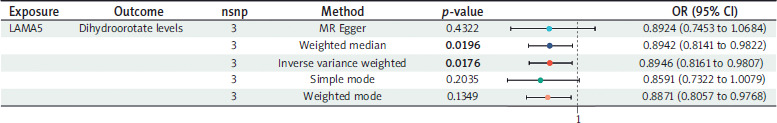
Using three SNPs to assess the causal relationship between LAMA5 and dihydroorotate levels, we observed a potential decrease in dihydroorotate levels associated with LAMA5 within the IVW method (OR = 0.8946, 95% CI: 0.8161–0.9807; p-value = 0.0176; heterogeneity = none; pleiotropy = pass; directionality = true). The weighted median and weighted mode analyses fully substantiated the inverse association, whereas the other two techniques showed a similar trend, though without reaching statistical significance. The detailed results are shown in Figure 5.
MR analysis between dihydroorotate levels and BC
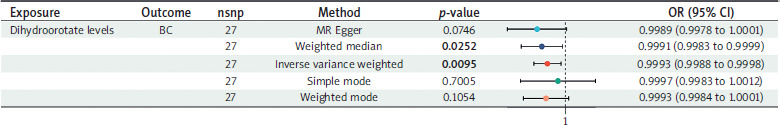
The causal effects of dihydroorotate levels on BC are shown in Figure 6. After adjusting for multiple testing, we identified 27 genetically representative SNPs associated with dihydroorotate levels. Using the IVW technique, we found that genetically predicted dihydroorotate levels were associated with a reduced risk of BC (OR = 0.9993, 95% CI: 0.9988–0.9998; p-value = 0.0095; heterogeneity = none; pleiotropy = pass; directionality = true). The direction of the effect estimates was consistent across the four different methods. All analyses demonstrated no evidence of heterogeneity or pleiotropy and passed the leave- one-out sensitivity test.
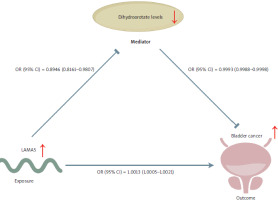
The conceptual framework underlying our research is illustrated in Figure 7. Based on the two-sample MR analysis, we further identified LAMA5 as a risk factor for BC. Our findings also indicated that LAMA5 decreased dihydroorotate levels, and conversely, lower dihydroorotate levels were associated with a reduced risk of BC. A definitive conclusion could be reached that the LAMA5 pathway might promote the development of BC through its influence on decreasing dihydroorotate levels.
Discussion
We performed an integrated MR analysis to investigate the associations between genes implicated in apoptosis- related genes, metabolites, and BC, with a particular emphasis on LAMA5, dihydroorotate levels, and their link to BC. Our findings indicated that LAMA5 was a risk factor for BC. Additionally, LAMA5 appeared to function as a suppressor of dihydroorotate levels, which in turn acted as an inhibitory factor for BC. Although the observed ORs for LAMA5 and dihydroorotate were modest, such effect sizes are consistent with the polygenic and multifactorial nature of BC, where disease pathogenesis arises from complex interactions between genetic and environmental factors. To further validate these findings, we conducted additional in vitro functional experiments. We confirmed the elevated expression of LAMA5 in BC cells. Subsequent in vitro assays revealed that the knockdown of LAMA5 significantly impacted cell proliferation, migration, and invasion in BC cells. Ultimately, it was determined that LAMA5 contributed to the risk of BC by enhancing tumor proliferation, migration, and invasion. From a metabolic perspective, its role might be associated with the reduction of dihydroorotate levels.
Apoptosis, a form of regulated cell death, is characterized by the activation of the caspase cascade following the release of cytochrome c from the mitochondria [23]. Apoptosis plays a significant role in the incidence and progression of BC and is a key target in biological processes and therapeutic interventions. The involvement of biological processes, such as the silencing of the caspase pathway and the down-regulation of the anti-apoptotic BCL2, is well documented in the etiology and progression of bladder cancer [24]. Mutations in FGFR3 enhanced the sensitivity of BC cells to apoptosis triggered by tumor necrosis factor–related apoptosis-inducing ligand, which might serve as a promising treatment for therapy [25]. Melatonin was found to inhibit growth and induced apoptosis in BC through the cyclin-dependent-like kinase pathway, suggesting its potential therapeutic application [26]. Through an integrated MR analysis, which was further supported by experimental validation, our study also identified LAMA5, an apoptosis-related gene, as a potential risk factor for BC, along with its metabolic mechanisms. Based on our findings, LAMA5 could serve as a therapeutic target for BC, warranting further in-depth investigation into the underlying mechanisms to fully understand its role in the disease.
The LAMA5 gene encodes a component of the laminin a chains, a family of glycoproteins residing in the basement membrane. It plays crucial roles in a spectrum of biological processes, such as cellular adhesion, cell differentiation, migration, signal transduction, neurite extension, and the spread of metastasis [27]. A novel missense mutation within the L4a domain of LAMA5 was identified, causing a marked decrease in the formation of the laminin 521 heterotrimer, which consequently led to the onset of nephrotic syndrome [28]. Researchers found that the interaction between LAMA5 and integrin b1 in ovarian cancer models dictated the compactness of cancer cell spheroids, with evidence from in vitro and in vivo experiments showing that blocking this interaction enhanced tumor invasiveness [29]. It was found that the LAMA5/ITGA4 axis is intricately associated with the transdifferentiation of pancreatic acinar to ductal cells, a pivotal event in the initiation of pancreatic cancer [27]. Our study concluded that LAMA5, identified as a risk factor for BC, exhibits significant overexpression in tumor cell lines, where it plays an inhibitory role in tumor cell growth, migration, and invasion. This gene has been confirmed as a potential therapeutic target for bladder cancer, with the relevant signaling pathways and targeted drugs awaiting further exploration.
Dihydroorotate is a crucial intermediate in pyrimidine nucleotide synthesis, undergoing oxidation by dihydroorotate dehydrogenase to form orotic acid, a rate-limiting step in de novo pyrimidine nucleotide production [30]. Researchers found that dihydroorotate levels were closely associated with the dynamic processes of postrenal acute kidney injury and, interestingly, exhibited a significant decrease in the context of takotsubo syndrome [31, 32]. Dihydroorotate levels have been shown to be crucially important in oncology, as evidenced by its development into an optimized diagnostic model for adrenal pheochromocytoma, demonstrating its potential as a clinical biomarker [33]. It was also found that tumor cells accumulate dihydroorotate under hypoxia, probably secreting nitrogen to prevent intracellular buildup, highlighting its key role in tumor metabolism and growth [34]. Based on the literature retrieved to date, there is no evidence to suggest that dihydroorotate levels change in typical cancers or its associated mechanisms. We are the first to identify dihydroorotate as a significant inhibitor for BC, as well as to propose its probable upstream gene, LAMA5.
The mechanisms underlying BC as well as its treatments have been extensively investigated for many decades, resulting in a multitude of theories. Han and colleagues [35] demonstrated that METTL3 positively regulates m6A modification, modulating the pri-miR221/222 process, which in turn promotes tumor proliferation in BC in vitro experiments. Analysis leveraging data from the TCGA database, combined with cellular functional experiments, revealed that HNRNPU influences the expression of neurofibromin 1, thereby promoting cisplatin resistance in BC [36]. Single- cell transcriptome analysis showed that histone lactylation activates transcription of target genes by enriching their promoter regions, which in turn triggers cisplatin resistance in BC [37]. Given the robustness of MR as a technique for exploring causal relationships in disease etiology [18], we employed this method and identified LAMA5 as a promoter and potential therapeutic target for BC, along with relevant metabolic mechanisms.
There were some advantages of our study. Firstly, we used MR techniques to establish the association between apoptosis-related gene, metabolite, and BC successfully. Furthermore, the methods circumvented the constraints such as financial and temporal expenditures and ethical concerns typically encountered in traditional observational studies and randomized controlled trials [11]. Lastly, our conclusions were further substantiated through validation in tissue samples and a comprehensive series of cellular function experiments, which provided more compelling evidence.
There were also many disadvantages. To begin with, the GWAS datasets used for our MR analysis were limited to individuals of European ancestry, which may restrict the extrapolation of our findings to other ethnic populations. Moreover, the data resources from GWAS were restrictive, preventing stratified analyses or adjustments for additional covariates, and there was a dearth of clinical data. Consequently, further high-quality clinical trials are essential to substantiate our findings. We used a more rigorous criterion to ensure a robust correlation with exposure and to eliminate linkage disequilibrium, thereby enhancing the precision of our study. This approach led to the identification of only a few SNPs for all our positive outcomes, which could potentially be subject to a modest inherent bias. Furthermore, our validation of LAMA5 expression in BC cells was limited to RNA levels, with DNA and protein levels yet to be explored, and the effects of LAMA5 on these cells were only evident when it was knocked down, with overexpression studies needed to fully elucidate its role. Lastly, our study was confined to MR, cellular phenotypes and in vitro functional experiments; therefore, in vivo validation and the identification of associated pathways remain necessary for further exploration.
Conclusions
LAMA5 was initially recognized as a risk factor for BC across three separate databases, comprising one discovery dataset and two validation datasets, and was found to augment cellular proliferation, migration, and invasion. Concurrently, we identified a specific metabolite, dihydroorotate, as a potential metabolic mechanism underlying the role of LAMA5 eQTL in the promotion of BC. Our research introduced a new target for BC and laid the groundwork for future investigations into LAMA5-related mechanisms in this disease.