Introduction
Acute myeloid leukemia (AML) is a hematologic malignancy characterized by clonal proliferation of immature myeloid cells, leading to bone marrow failure and severe immunosuppression. Infectious complications are frequent in AML patients, often exacerbating their clinical course and significantly increasing mortality risk. The identification of reliable biomarkers for infection severity and outcomes in this population is crucial for guiding clini-cal management and improving survival rates.
Interleukin-6 (IL-6), a pro-inflammatory cytokine, has gained attention for its role in mediating inflammatory responses and its association with severe infections and adverse clinical outcomes. Elevated IL-6 levels have been linked to poor prognosis in various infectious and non-infectious conditions, including bacterial sepsis and cancer. In AML, the relationship between IL-6 and infection-related outcomes remains underexplored, yet emerging evidence suggests that IL-6 could play a central role in driving immune dysregulation and influencing patient survival. The purpose of this study is to evaluate the prognostic utility of IL-6 in predicting infection severity and mortality in AML patients with infectious complications [1, 2].
Material and methods
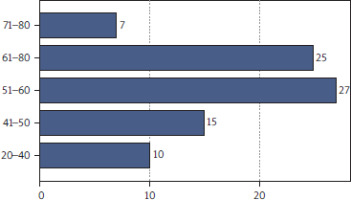
In this retrospective study, we analyzed a cohort of 84 AML patients who developed infectious complications during their treatment (Figure 1). At the onset of infection, levels of IL-6, C-reactive protein (CRP), and procalcitonin (PCT) were measured as key biomarkers to assess infection severity and predict clinical outcomes. The severity of infections was graded on a scale from 1 to 5, with grade 5 representing the most severe and often fatal cases. Serum IL-6 levels were quantified using an enzyme-linked immunosorbent assay (ELISA), whereas CRP and PCT concentrations were measured with standard automated immunoassays in a certified clinical laboratory. Biomarker levels were assessed at the time of infection diagnosis and monitored throughout the course of the infection. Demographic data, including age and gender, were also collected to evaluate potential correlations between patient characteristics and biomarker levels.
Figure 1
Patient demographics by age. The distribution of patients across age groups is shown, with the highest representation in the 51–60 and 61–70 age groups
Statistical analysis
Statistical analysis included descriptive measures to summarize the distribution of infection severity and biomarker levels across different patient groups. The correlation between IL-6, CRP, PCT, and infection severity was examined using Spearman’s rank correlation, revealing significant associations, particularly with IL-6.
All statistical analyses were performed using SPSS software. To further investigate the predictive power of these biomarkers, one-way analysis of variance (ANOVA) was performed to compare IL-6, CRP, and PCT levels across the different infection severity grades. Additionally, multivariate logistic regression was employed to assess the influence of IL-6, alongside demographic factors and hematological parameters, on mortality risk.
Results
The infections occurring in AML patients were graded from 1 to 5 based on severity, with 60.71% of cases classified as grade 3. Initial biomarker analysis revealed notable variations in levels of IL-6, CRP, and PCT across different infection grades. Given the known role of these markers in inflammation and infection, we sought to determine which one could most reliably predict infection severity and patient outcomes.
At first, both CRP and PCT demonstrated increases in response to infection, consistent with their established roles as markers of inflammation. CRP showed a steady rise in patients with moderate to severe infections, suggesting its potential as a predictor of worsening clinical conditions. PCT followed a similar pattern, with levels rising as infections progressed to more critical stages. These initial findings seemed to position both CRP and PCT as reliable markers of infection severity, especially in cases categorized as grade 3 and above.
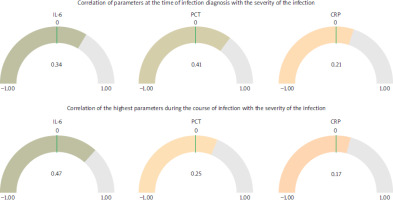
The analysis revealed significant differences in the behavior of biomarkers IL-6, PCT, and CRP in relation to infection severity and mortality among AML patients. At the time of infection diagnosis, PCT showed the strongest correlation with infection severity (r = 0.41), followed by IL-6 (r = 0.34) and CRP (r = 0.21). However, as the infection progressed, IL-6 demonstrated a marked increase in predictive value, showing the highest correlation with severity (r = 0.47), while PCT and CRP exhibited weaker associations (r = 0.25 and r = 0.17, respectively). This suggests that while PCT may be useful for initial diagnosis, IL-6 is more reliable for predicting infection outcomes during its progression (Figure 2).
Figure 2
Correlation of biomarkers with infection severity. The correlation coefficients between IL-6, PCT, CRP, and infection severity are presented. At diagnosis, PCT shows the strongest correlation, while IL-6 surpasses other markers during infection progression
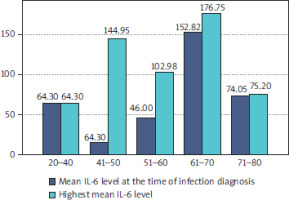
IL-6 levels varied significantly across different age groups. Patients aged 61–70 years exhibited the highest mean IL-6 levels at both the time of diagnosis (152.82 pg/ml) and during the course of infection (178.75 pg/ml). In contrast, patients aged 41-50 years showed the lowest IL-6 levels at diagnosis (14.90 pg/ml) but experienced a dramatic increase during infection progression, reaching 144.05 pg/ml. This suggests that while IL-6 levels may initially be low in this group, the rapid escalation could indicate a heightened risk for severe outcomes. Patients aged 20–40 years and 71–80 years had relatively stable IL-6 levels, with minimal increases during the infection course, indicating potentially lower susceptibility to severe infections (Figure 3).
Figure 3
IL-6 levels across age groups. Mean IL-6 levels at diagnosis and during infection progression are shown for different age groups
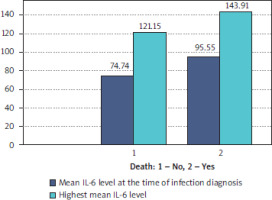
IL-6 levels were also strongly associated with patient mortality. Patients who survived had mean IL-6 levels of 74.74 pg/ml at diagnosis and 121.15 pg/ml at the peak of infection. In contrast, non-survivors exhibited significantly higher levels, with 95.55 pg/ml at diagnosis and 143.91 pg/ml during infection progression. This stark difference highlights the prognostic value of IL-6 in predicting mortality risk, with an odds ratio of 2.45 (95% CI: 1.80–3.33) for elevated IL-6 levels (Figure 4).
In summary, IL-6 emerged as the most reliable biomarker for predicting infection severity and mortality in AML patients. Its superiority over traditional markers such as CRP and PCT was particularly evident during the progression of infection, making it an invaluable tool for early risk stratification and personalized intervention planning.
Therefore IL-6 was found to be a more powerful predictor of adverse outcomes in AML patients with infections than both CRP and PCT.
Discussion
Our findings reinforce the growing recognition of IL-6 as a critical biomarker for infection severity and mortality in AML patients. The dramatic elevation of IL-6 during severe infections suggests that this cytokine not only reflects the intensity of the inflammatory response but may also contribute to the pathogenesis of infection-related complications in this population. This is consistent with previous research that links high IL-6 levels to worse outcomes in other inflammatory conditions, such as sepsis and chronic inflammatory diseases [3, 4].
Moreover, the pronounced IL-6 increases observed in middle-aged patients (41–60 years) highlight the need for targeted interventions in this demographic. Given the high mortality risk associated with IL-6 surges in this age group, therapeutic strategies aimed at modulating IL-6 activity, such as IL-6 receptor antagonists (e.g., tocilizumab), may offer a promising avenue for improving patient outcomes [2, 5, 6].
While other biomarkers, including CRP and PCT, have demonstrated utility in assessing infection severity, the superior predictive value of IL-6 observed in our study positions it as a key tool in clinical decision-making for AML patients. Its ability to more accurately predict mortality risk compared to traditional markers reinforces the importance of incorporating IL-6 monitoring into standard care protocols for AML patients with infectious complications [6, 7].
Conclusions
The present analysis underscores the prognostic signi-ficance of IL-6 in predicting infection severity and mortality in AML patients. Routine monitoring of IL-6 levels could enable earlier identification of high-risk individuals, facilitating more timely and personalized interventions aimed at reducing infection-related mortality. In particular, middle-aged AML patients with rapidly increasing IL-6 levels should be closely monitored and considered for therapeutic strategies that target IL-6 to mitigate the risk of fatal outcomes [3, 7, 8].
As IL-6 continues to emerge as a pivotal biomarker in the management of infectious complications, further research is needed to explore its diagnostic potential and to refine clinical guidelines for its use in routine care. The incorporation of IL-6 monitoring into standard practice has the potential to improve infection management in AML patients.