Introduction
Infective endocarditis is a severe condition that poses unique challenges in the pediatric population, particularly among those with congenital heart defects and prosthetic materials. Fungal infections, primarily caused by Candida species, are estimated to occur in 0.2–0.35% of all infective endocarditis cases but are more frequent in patients with prosthetic implants, where the incidence ranges from 2% to 4% [1]. These infections are associated with high mortality rates, reported to be between 39% and 100% [2, 3]. The significant mortality reflects both the intrinsic virulence of fungal pathogens and the challenges associated with timely and accurate diagnosis. Compared to bacterial pathogens, fungi such as Candida glabrata demonstrate a unique ability to form biofilms on prosthetic surfaces, which protects them from both host immune responses and antifungal agents, making eradication particularly difficult.
We present a case of invasive fungal infection in a 5-month-old infant with tetralogy of Fallot (TOF) after repeated percutaneous right ventricular outflow tract (RVOT) stenting. We explore the diagnostic and therapeutic complexities and the role of multidisciplinary collaboration in achieving a successful outcome.
Case report
The patient was a 5-month-old male with TOF with severe sub-valvular pulmonary stenosis, a right-sided aortic arch, and coarctation of the aorta. He was born prematurely at 35 weeks of gestation as part of a twin pregnancy. The infant had a normal thymus but he exhibited dysmorphic features, including preauricular tags, cleft lip, suspected submucosal cleft palate, and vertebral anomalies consistent with a potential diagnosis of Goldenhar syndrome. These features complicated the overall clinical management, necessitating a multidisciplinary approach.
The patient had undergone multiple prior interventions at another hospital, including stent (Palmas Genesis 6 × 18 mm) implantation in the RVOT, balloon angioplasty for aortic coarctation, and placement of a left Blalock-Taussig shunt (LBTS) to improve pulmonary blood flow. Despite these procedures, the child presented to the emergency department with profound cyanosis suggesting decreased pulmonary blood flow.
Echocardiography demonstrated significant narrowing at the site of the RVOT stent, accompanied by severe stenosis of the LBTS and mild residual coarctation of the aorta. The patient underwent urgent cardiac catheterization to relieve the obstructions (Figures 1 A, B). The successful balloon angioplasty of the LBTS was performed using a 3.5 × 15 mm catheter (Ryurei, Terumo; Figure 1 C), and the coarctation was dilated with 6 mm and 8 mm balloons (Powerflex; Cordis). Due to critical narrowing and fractures within the previously implanted RVOT stent, access from the ventricular side was severely compromised. Therefore, a new 4 × 18 mm stent (Multi-Link, Abbott) was implanted via the LBTS to address the obstruction and reinforce the fractured stent (Figure 1 D). None of the devices used during the interventional procedures were drug-eluting. The cardiac catheterization procedure was uncomplicated. However, during the first 24 h in the intensive care unit, the patient experienced episodes of bradycardia and hypoxemia, requiring brief resuscitation, reintubation and inotropic support.
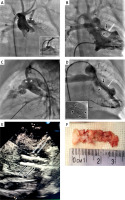
Figure 1
Imaging and surgical findings in a neonate with tetralogy of Fallot after repeated right ventricle outflow tract stenting complicated by invasive fungal infection. A – Initial aortography shows a right aortic arch, aortic coarctation (black arrowhead), and a stenosed left sided Blalock-Taussig shunt (L-BTS) at the pulmonary end (white arrowhead). B – Initial right ventricular angiography reveals severely reduced flow within the fractured (black arrows) previously implanted stent (white arrow) in the RVOT. C – Angiography from the left subclavian artery after balloon angioplasty of the L-BTS (3.5 × 15 mm Ryurei balloon, Terumo) shows effective dilation of the stenosed pulmonary end anastomosis (white arrowhead). D – Angiography after implantation of a 4 × 18 mm Multilink stent (Abbott) into the fractured stent in the RVOT shows improved wide flow (white arrow). Black arrows indicate the site of fracture of the previously implanted, now covered by the new stent. Inset in the lower left corner – close-up of the stents in the RVOT: black arrows point to the fracture site of the previously implanted stent, now covered by the new stent; black arrowheads indicate the proximal segment of the new stent, intentionally implanted slightly proximal to the previous stent to enhance inflow and widen the muscular stenosis at this level. E – Echocardiography: black arrows indicate stents implanted in the RVOT; white arrows point to linear echogenic structures along the anterior edge of stents. F – Intraoperative photograph showing stents after excision from the RVOT
Laboratory investigations revealed markedly elevated inflammatory markers, including a C-reactive protein (CRP) level of 222 mg/l and a procalcitonin (PCT) level of 1.89 ng/ml, raising concern for sepsis. Blood cultures confirmed the growth of Candida glabrata, a fungal pathogen known for its resistance to first-line antifungal agents. The child underwent immunological consultation, and normal immunoglobulin levels and lymphocyte subpopulations were found. Initial treatment with fluconazole was initiated but was quickly escalated to micafungin after susceptibility testing revealed resistance. Despite appropriate antifungal therapy, the clinical course remained challenging, with persistently elevated circulating Candida antigen levels and worsening hepatic dysfunction (elevated AST, ALT, GGT levels).
Echocardiography identified linear echogenic structures along the anterior edge of the newly implanted stent, consistent with potential vegetations (Figure 1 E). Additional imaging with computed tomography of the chest and abdomen failed to identify other infectious foci.
Due to persistent antigenemia and clinical suspicion of fungal endocarditis, antifungal therapy was switched to liposomal amphotericin B, administered for 6 weeks. During this time, the patient’s clinical condition gradually stabilized, with a decline in inflammatory markers and Candida antigen levels. To further evaluate for residual infection, positron emission tomography with 18F-fluorodeoxyglucose (FDG-PET/CT) was performed 3 months after the intervention. This imaging modality revealed no metabolically active areas suggestive of ongoing infection, supporting the decision to discontinue antifungal therapy.
The decision to definitively correct the defect was postponed due to low body weight, the presence of a vessel crossing the RVOT (an additional left anterior descending artery originating from the right coronary artery) and the complicated postoperative course. At 16 months of age, the patient underwent definitive corrective surgery for TOF. Preoperative blood cultures and fungal antigen tests were negative. Prophylactic antifungal therapy with anidulafungin was administered perioperatively to prevent relapse. The complete antifungal therapy is summarized in Table I. Intraoperative findings revealed no evidence of active infection, and cultures of the removed RVOT stent were negative for fungal growth (Figure 1 F). The postoperative course was uneventful, and the patient was discharged in a stable condition.
Table I
Summary of the antifungal therapy in an infant with tetralogy of Fallot after repeating right ventricle outflow tract stenting and invasive fungal infection
| Type of procedure | Age | Treatment and dosage | Duration of treatment | Circulating Candida antigen | CRP (N < 5 mg/l) |
|---|---|---|---|---|---|
| Cardiac catheterization | 6 months | Fluconazole 6 mg/kg/day OD | 4 days | 145 pg/ml Positive blood culture Candida glabrata | 222 mg/l |
| 6 months | Micafungin 1.8 mg/kg, OD | 10 days | 270 pg/ml | < 5 mg/l | |
| 6.5 months | Micafungin 3.6 mg/kg, OD | 14 days | 296 pg/ml | < 5 mg/l | |
| 7–8 months | Liposomal amphotericin B 45 mg/m2/day, OD | 42 days | 263 pg/ml (1st*) | < 5 mg/l | |
| 154 pg/ml (7th*) | |||||
| 99 pg/ml (14th*) | |||||
| 8–13 months | Posaconazole 12 mg/kg/day TID | 157 days | Negative (4th*) | < 5 mg/l | |
| Negative (38th*) | |||||
| Negative (94th*) | |||||
| Negative (151st*) | |||||
| 13–16 months | No treatment | Not performed | Not performed | ||
| TOF correction | 16 months | Anidulafungin 3 mg/kg/day OD | 3 days | Not performed | < 5 mg/l |
| 16–17 months | Anidulafungin 3 mg/kg/day OD | 21 days | Negative (2nd***) | 192 mg/l | |
| Negative (17th***) | 38 mg/l | ||||
| Negative (20th ***) | 19 mg/l |
Discussion
Differentiating invasive fungal infections from non-invasive fungal infections with associated endocarditis or infections of stents or other artificial materials can be difficult. Given the ability of fungi to form biofilms, colonization of artificial surfaces must always be considered.
In the presented patient, a sudden deterioration in general condition with shock occurred on the first postoperative day after an interventional procedure. During this procedure, a new stent was implanted and expanded with a balloon catheter within the ruptured and narrowed stent in the RVOT. The boy, previously treated at another center, had not been tested for fungal infections before. Considering the rapid clinical response after the procedure and the rapid growth of Candida glabrata in cultures, the possibility of colonization of the old stent material by the identified fungi was considered. Available microbiological tests were not sufficient to determine whether the implant was colonized or covered with fungal biofilm.
Imaging diagnostics of the endocardium, stents, and other implanted materials in patients with congenital heart defects is challenging, particularly in structures of the right heart. Transthoracic echocardiography (TTE) is the primary method for diagnosis and monitoring of the disease. Its sensitivity in the pediatric population is significantly higher than in adults, reaching up to 97% in children [4]. However, in patients with implanted artificial materials after cardiac procedures, the sensitivity of this examination significantly decreases, with up to 70% of examinations in such groups yielding false-negative results [5].
In this patient’s case, the TTE result was inconclusive: additional hyperechoic structures projecting into the lumen of the stent were observed, suggesting vegetations, though they were not as large as those described in fungal vegetations in the literature. For patients with artificial materials in the right heart, the possibility of false-positive results due to artifacts or thrombi indicates the need for additional imaging methods [6]. A CT scan can reveal the presence of vegetations or peri-valvular changes that meet the major criteria for diagnosing infective endocarditis according to the Duke-ISCVID 2023 criteria, or vascular changes such as septic emboli or abscesses, which meet the minor criteria. In our patient, full-body CT was performed, but no changes suggestive of infective endocarditis were observed. Given the persistent inconclusive clinical picture, we decided to extend the diagnostic workup with 18F-FDG PET/CT. This imaging modality significantly increases the sensitivity for diagnosing infective endocarditis, particularly in patients with artificial heart valves [7]. A potential limitation of this test is the presence of inflammation around implanted materials for up to 3 months after surgery, which may result in false-positive findings.
After completing therapy with liposomal amphotericin B, stabilizing the patient, normalizing test results, and sterilizing blood cultures, a multidisciplinary team of a cardiologist, cardiac surgeon, infectious disease specialist, and microbiologist decided to initiate maintenance therapy with posaconazole until further results could be obtained. The 18F-FDG PET/CT was performed 3 months after the last cardiac catheterization, and no metabolically active inflammatory processes were detected. During this period, we also failed to achieve therapeutic levels of posaconazole despite multiple dose adjustments and changes in the method of administration by the parents. Consequently, therapy was discontinued. Before the final correction procedure, a comprehensive evaluation for fungal infection was conducted, with all results being negative. Sterilization of blood cultures during therapy does not guarantee that infection will not recur from the colonized site once treatment is stopped. The circulating Candida antigen, which remained positive for a long time in this patient without showing a downward trend, is also not a reliable marker of active fungal infection [8].
Given the planned intervention at the previously suspected site of infection (the stent), prophylaxis with anidulafungin was employed. The procedure was uneventful.
Conclusions
Despite severe prognosis associated with invasive fungal infection, suspected stent infection, and the presence of artificial material, the patient’s treatment was completed successfully. Planning appropriate management, whether surgical or conservative, including the choice of antifungal agent, duration of therapy, and decisions regarding maintenance therapy, is often only possible through individualized, multidisciplinary consultations involving a cardiologist, cardiac surgeon, infectious disease specialist, and microbiologist.








